Figures & data
Figure 1 Study schematic.
Abbreviations: ICS, inhaled corticosteroid; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; SABA, short-acting β-agonist.
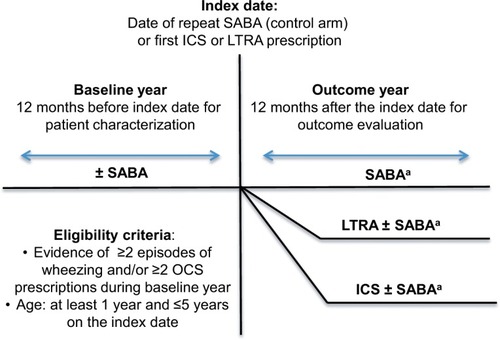
Table 1 Baseline year characteristics of children included in the controller (ICS or LTRA) vs SABA matched cohort comparisons
Figure 2 Forest plot depicting OR (95% CIs) of wheezing/asthma attack for the four matched cohort comparisons.
Abbreviations: EF, extrafine; ICS, inhaled corticosteroid; LTRA, leukotriene receptor antagonist; SABA, short-acting β-agonist.
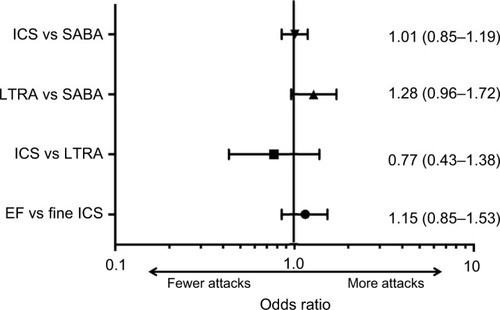
Figure 3 Percentage of children with one or more wheezing/asthma attacks during the baseline year (before the first prescription of ICS, LTRA, or repeat SABA) and during the outcome year in the four matched cohort comparisons: (A) ICS ± SABA vs SABA, (B) LTRA ± SABA vs SABA, (C) LTRA vs ICS, and (D) EF-particle ICS vs fine-particle ICS.
Abbreviations: ED, emergency department; EF, extrafine; ICS, inhaled corticosteroid; LTRA, leukotriene receptor antagonist; OCS, oral corticosteroid; SABA, short-acting β-agonist.
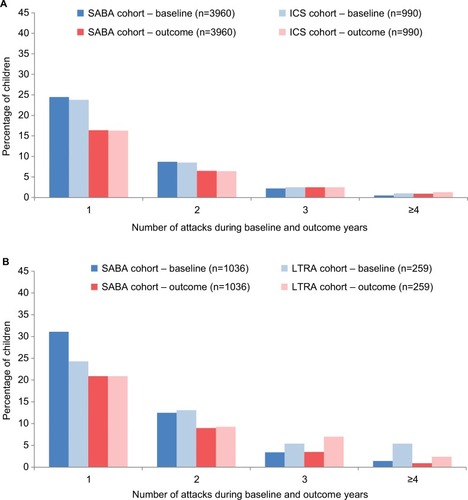
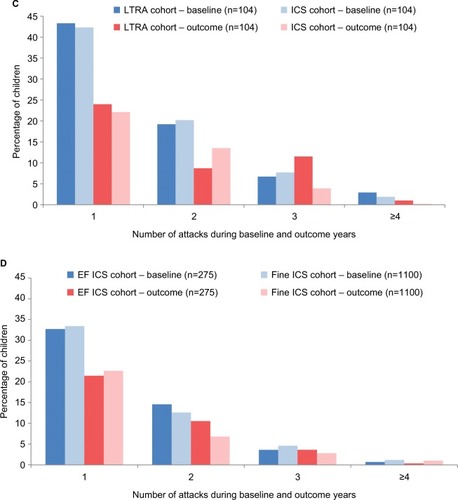
Table 2 Outcome measures during 1 follow-up year for the controller (ICS or LTRA) vs SABA matched cohort comparisons
Table 3 Baseline year characteristics of children included in the two controller therapy matched cohort comparisons: LTRA vs ICS and EF-particle ICS vs fine-particle ICS
Table 4 Outcome measures during 1 follow-up year for the controller therapy matched cohort comparisons: LTRA vs ICS and EF-particle ICS vs fine-particle ICS
