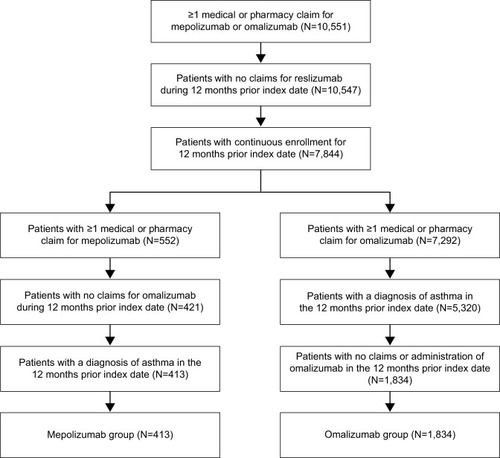
Figures & data
Table 1 Demographic characteristics at index date
Table 2 Clinical characteristics of patients during the 12 months prior to treatment
Figure 2 Proportion of patients with exacerbations during the (A) 3-month and (B) 12-month baseline period.
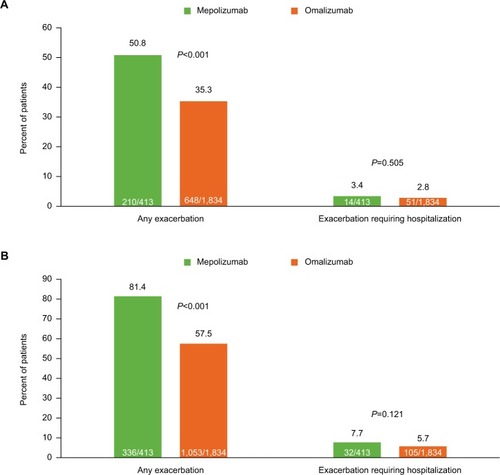
Figure 3 Proportion of patients with an asthma-related HCRU during the 12-month baseline period.
Abbreviations: ER, emergency room; HCRU, healthcare resource utilization.
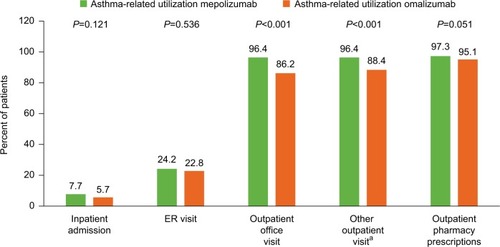
Table 3 Asthma-related HCRU during the 12-month baseline period
Figure 4 Asthma-related (A) and total (B) healthcare expenditure during the 12-month baseline period.
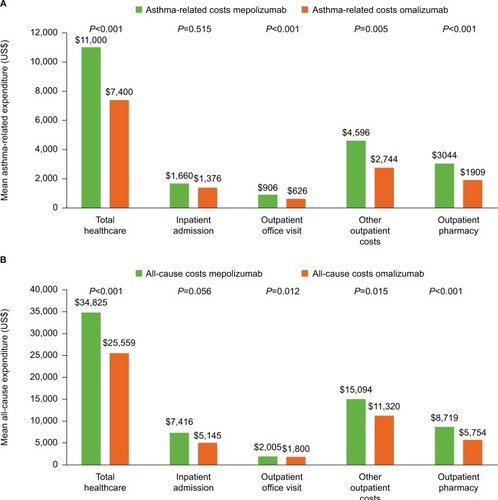
Figure S1 Proportion of patients with all-cause HCRU during the 12-month baseline period (main analysis excluding patients with prior biologic use).
Notes: aIncludes other outpatient services, such as radiology services, laboratory tests, and outpatient infusion.
Abbreviations: ER, emergency department; HCRU, healthcare resource utilization.
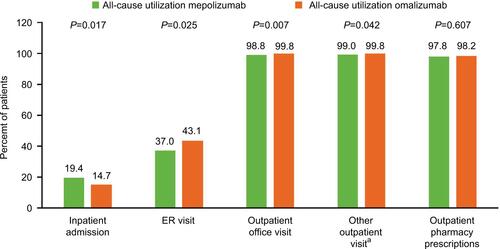
Figure S2 Proportion of patients with exacerbations during the 12-month baseline period (sensitivity analysis including patients who had received omalizumab or mepolizumab during the baseline period).
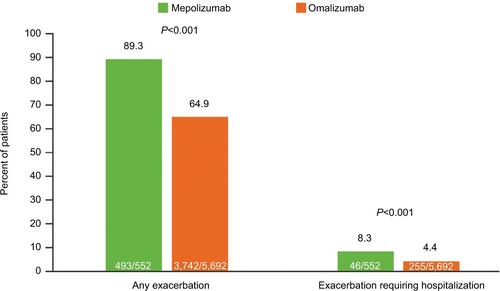
Figure S3 Proportion of patients with an asthma-related HCRU during the 12-month baseline period (sensitivity analysis including patients who had received omalizumab or mepolizumab during the baseline period).
Abbreviation: ER, emergency room; HCRU, healthcare resource utilization.
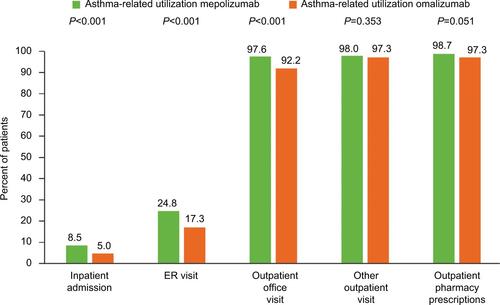
Figure S4 Asthma-related (A) and total (B) healthcare expenditure during the 12-month baseline period (sensitivity analysis including patients who had received omalizumab or mepolizumab during the baseline period).
Abbreviation: ER, emergency room.
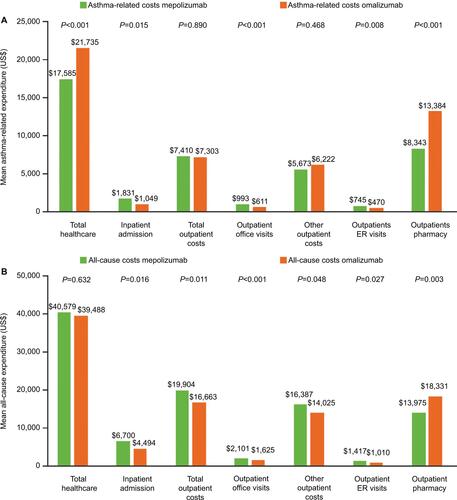
Table S1 All-cause HCRU during the 12-month baseline period (main analysis excluding patients with prior biologic use)
Table S2 Demographic characteristics at index date (sensitivity analysis including patients who had received omalizumab or mepolizumab during the baseline period)
Table S3 Clinical characteristics of patients during the 12 months prior to treatment (sensitivity analysis including patients who had had received omalizumab or mepolizumab during the baseline period)
Table S4 Asthma-related HCRU during the 12-month baseline period (sensitivity analysis including patients who had received omalizumab or mepolizumab during the baseline period)
Data availability
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data, and for clinical studies not listed, please submit an enquiry via the website.