Figures & data
Figure 1 Benralizumab 2-Year Integrated Analysis Study Design.
Figure 2 Trial profile for benralizumab 2-year integrated analysis. aIncluding patients who discontinued treatment but attended all study visits. bOne patient did not receive treatment and one patient did not fulfill eligibility criteria.
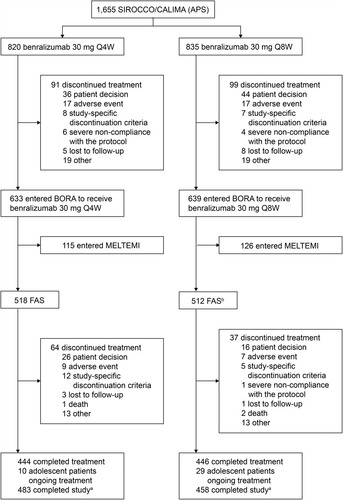
Table 1 Demographics And Baseline Clinical Characteristics Of Benralizumab 2-Year Integrated Analysis Patients (FAS)a
Figure 3 Annual asthma exacerbation rate for patients in SIROCCO/CALIMA pivotal study (Full analysis set, on-treatment period, blood eosinophil counts ≥300 cells/µLa). n values are 323/318 for patients receiving benralizumab 30 mg Q4W and Q8W, respectively. Baseline value represents exacerbation rate over the year before pivotal study entry. aBlood eosinophil counts at baseline of preceding pivotal studies.
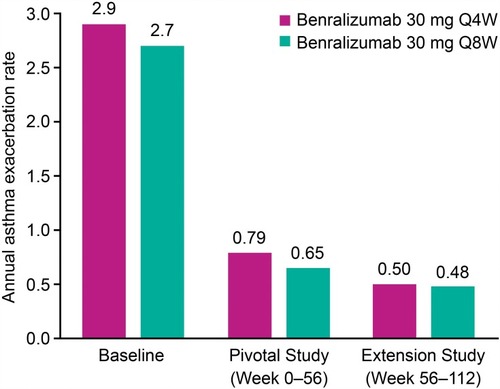
Table 2 Asthma Exacerbations For SIROCCO/CALIMA Pivotal Study Patients In The Benralizumab 2-Year Integrated Analysis (FAS, On-Treatment period)
Figure 4 Change from SIROCCO/CALIMA pivotal study baseline in (A) Lung Function (FEV1), (B) ACQ-6, and (C) AQLQ(S)+12 with benralizumab during the 2-year integrated analysis (Full analysis set, on-treatment period, blood eosinophil counts ≥300 cells/µLa). Error bars represent standard error. aBlood eosinophil counts at baseline of preceding pivotal studies.
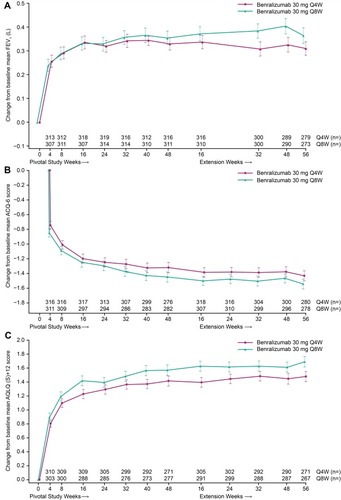
Table 3 Changes In Lung Function, ACQ-6, And AQLQ(S)+12 With Benralizumab During The 2-Year Integrated Analysis Period For SIROCCO/CALIMA Pivotal Study Patients (FAS, On-Treatment period)
Table 4 Adverse Events, Injection-Site Reactions, And Hypersensitivity For SIROCCO/CALIMA Pivotal Study Patients During The 2-Year Integrated Analysis Period (FAS, On-Treatment Period, All Patients Regardless Of Blood Eosinophil Count)
