Figures & data
Figure 1 Alpha-Gal syndrome (AGS). (A) Sensitization after several tick bites. Tick saliva contains glycoproteins, glycolipids with α-Gal epitopes and other unknown salivary biomolecules that could be involved in the pathology of AGS. The glycan α-Gal is presented to T helper 2 (Th2) cells through antigen-presenting cells (APCs) as dendritic cells, macrophages or even B cells. Once T cells are activated, B cells are leading to produce IgE against α-Gal (anti α-Gal-IgE) in an enriched interleukin environment and potentiate IgE production in plasma cells. Free IgE are now available to interact and bind to IgE receptors present in basophils and mast cells. (B) Allergic Reaction. When AGS patients ingest mammalian meat containing α-Gal bound to proteins or lipids, these molecules expressing the allergen epitope are absorbed and incorporated to lipid/protein macromolecules during digestion (chylomicrons, lipoproteins), which will be processed and transport through protein or lipid metabolism to systemic circulation and peripheral tissues. About 3–6 hours post-consumption, IgE-mediated and coated effectors will recognize the allergen, leading to degranulation of basophils and mast cells and promoting a systemic delayed allergic reaction. AGS can also comprise an immediate anaphylactic reaction, triggered using α-Gal containing drugs, administered via parenteral due to therapeutic reasons.
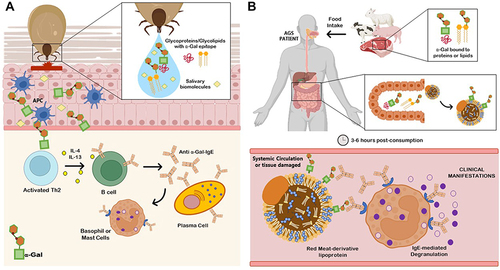
Figure 2 Conventional and next generation methods for the diagnosis of the alpha-Gal syndrome (AGS).
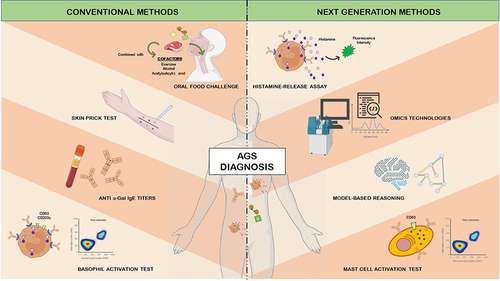
Table 1 Drugs and Associated Pharmacological Class Used for α-Gal Syndrome Medical Treatment
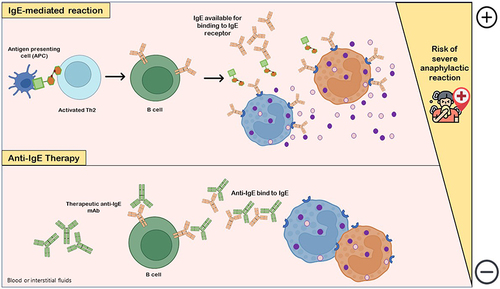
Figure 3 Anti-IgE therapy. IgE-mediated reaction with release of histamine and other co-factors occurs due to interaction of allergen-specific IgE available with IgE receptor in mediator cells (basophils, eosinophils or mast cells), which are degranulated and increase the risk of life-threatening anaphylactic adverse reactions. The pharmacological and clinical aims of the use of anti-IgEs monoclonal antibodies (mAbs) as drugs is to downregulate and/or decrease IgE production by B cells. Anti-IgE antibodies bind to both IgE-expressing B cells and free serum IgE, markedly decreasing IgE levels available for binding to IgE receptor in allergic reaction-mediator cells and, consequently, gradually compromising mast cells and basophils sensitivity to allergens.