Figures & data
Table 1 Inclusion Criteria, Exclusion Criteria, and Contraindications for Wheat Challenge
Figure 1 Summary of modified 3-day challenge protocol.
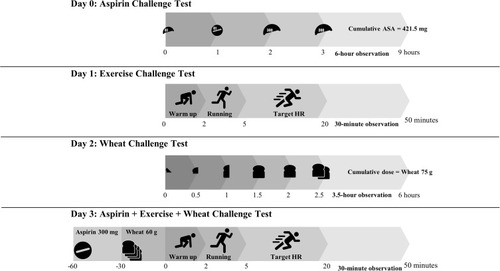
Figure 2 Flow diagram of patient recruitment.
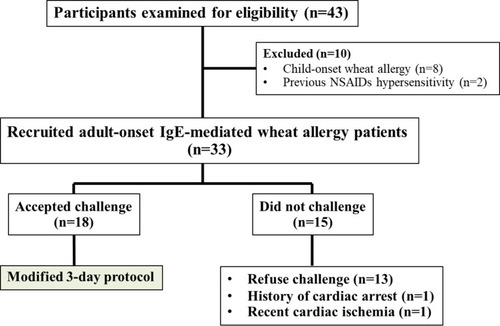
Table 2 Clinical Characteristics of 33 Patients in Adult-Onset Wheat Allergy Cohort
Table 3 Clinical Characteristics of Patients Who Underwent Challenge Procedure
Figure 3 Outcomes of modified 3-day challenge protocol.
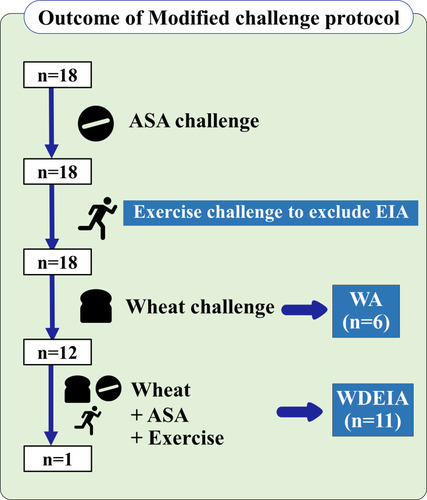
Table 4 Comparison of Wheat-Cofactor Challenge Protocols in Patients with Wheat Allergy
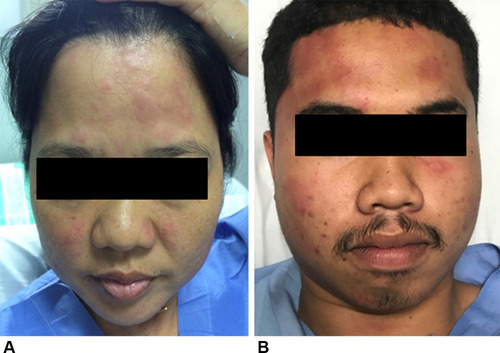
Figure 4 Isolated facial urticaria observed during the challenge in patients with adult-onset IgE-mediated wheat allergy. (A) A wheat-allergic patient during the open wheat challenge (day 2). (B) A wheat-dependent exercise-induced anaphylaxis patient during the combined wheat-cofactors challenge (day 3).