Figures & data
Figure 1 Eosinophilic esophagitis: clinical & pathophysiologic overview.
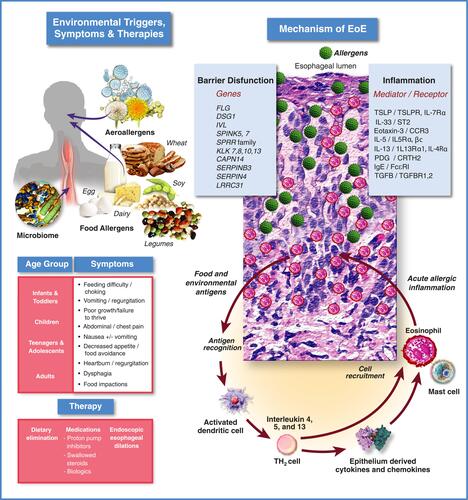
Figure 2 Progression of PPI therapy from diagnostic tool to therapy for EoE. Initial belief that EoE was a consequence of GERD led to early interest in PPIs as a therapy for EoE. Next, it was hypothesized that PPI-responsive esophageal eosinophilia (PPI-REE) was a condition distinct from EoE. A lack of response to PPIs was subsequently viewed as an essential diagnostic criterion for EoE. Subsequently, characterizations of PPI-REE and EoE patients at the molecular level showed that the two conditions are virtually identical leading to the hypothesis that they are at different points along a continuum. Recent guidelines, enlightened by this observation, now view PPIs as a therapy rather than a diagnostic for EoE.
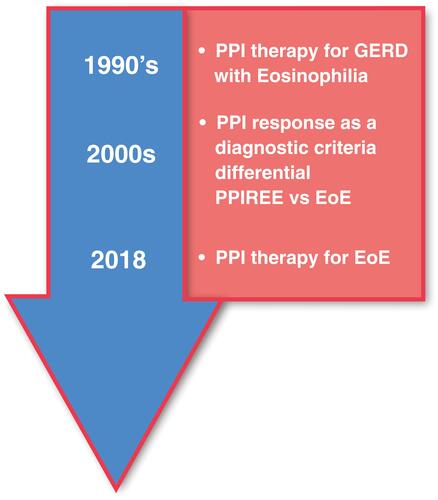
Figure 3 Proposed mechanisms of PPI efficacy for EoE. (A) Anti-Secretory Mechanism: Hypothesizes that the integrity of the esophageal epithelium is compromised by exposure to gastric acid leading to ingress of antigens and activation of an immune response. Acid suppression by PPIs allows the esophageal epithelium to heal facilitating resolution of inflammation. (B) Anti-Inflammatory Mechanisms: 1.) PPIs block expression of cell surface adhesion molecules, inhibiting migration of inflammatory cells to the esophageal epithelium; 2.) PPIs block STAT6 mediated expression of eotaxin-3 reducing recruitment of eosinophils to the esophageal epithelium; 3.) PPIs can stimulate the aryl hydrocarbon receptor normalizing expression of genes involved in barrier function including, filaggrin, loricrin, and involucrin through inhibition of the IL-4/IL-13-STAT6 pathway; 4.) PPIs can inhibit the activity of ATP12A, the non-gastric P2-type H+, K+-ATPase. IL-4 mediated induction of eotaxin-3 secretion is sensitive to inhibition of ATP12A.
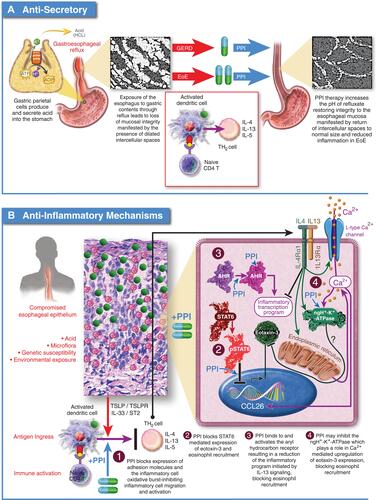
Figure 4 PPI efficacy in adults: histologic remission (<15 eos/hpf). The analysis was conducted using the R statistical package metafor,Citation210 assuming a fixed effects model and using inverse-variance weighting. The reported summary statistic is the back-transformed inverse-variance weighted average for histologic remission across all studies listed in adults. References: Garrean, 2009,Citation211 Peterson, 2010,Citation104 Molina-Infante, 2011,Citation22 Abe, 2011,Citation119 Fujiwara, 2012,Citation108 Francis, 2012,Citation109 Vazquez-Elizondo, 2013,Citation110 Moawad, 2013,Citation105 Lee, 2013,Citation122 Dellon, 2013,Citation107 Mangla, 2014,Citation212 Molina-Infante, 2014,Citation98 Van Rhijn, 2014,Citation55 Philpott, 2016,Citation113 Gómez-Torrijos, 2016,Citation112 Frazzoni, 2021Citation125.
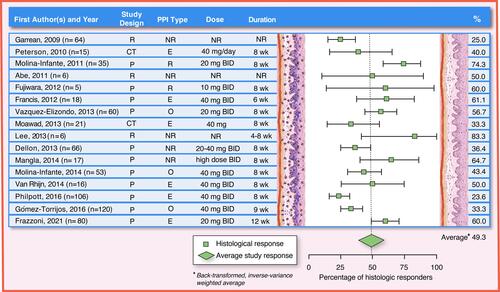
Figure 5 PPI efficacy in children: histologic remission (<15 eos/hpf). The analysis was conducted using the R statistical package metafor,Citation210 assuming a fixed effects model and using inverse-variance weighting. The reported summary statistic is the back-transformed inverse-variance weighted average for histologic remission across all studies listed in children. References: Sayej, 2009,Citation21 Dranove, 2009,Citation20 Schroeder, 2013,Citation185 Rea, 2013, Gutiérrez-Junquera, 2016,Citation187 Gómez-Torrijos, 2018,Citation188 Harris, 2018,Citation213 Vieira, 2020,Citation189 Rosen, 2021Citation214.
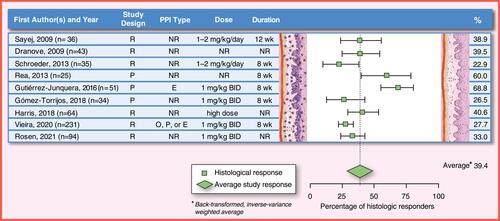
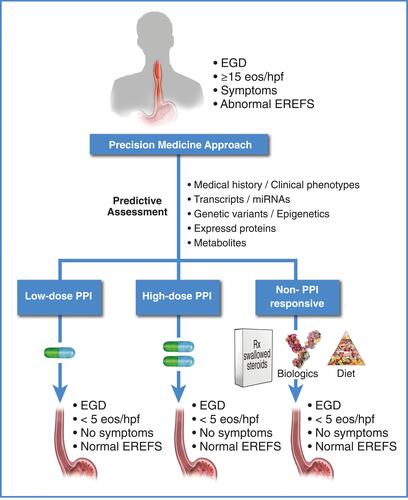
Figure 6 Therapy for eosinophilic esophagitis: framework for proposed future directions. Current research is focused on identifying a minimal set of non-invasive informative markers (transcriptomic,Citation27,Citation126,Citation215 genomic,Citation3 proteomic,Citation216 metabolomic, history, etc.) that predict how a patient will respond to PPIs for EoE. For a review of potentially informative non-invasive biomarkers that predict active EoE, see Votto et al.Citation217 Some of the biomarkers reviewed by Votto et al may also be informative for a PPI-responsive outcome when assessed prior to PPI therapy. Given this information, patients can potentially be identified as low-dose PPI responders, high-dose PPI responders, PPI non-responders, etc., prior to initiation of therapy, allowing selection of the appropriate therapy to achieve resolution of inflammation.