Figures & data

Figure 1 TSLP Acts Across the Spectrum of Asthma Inflammation. TSLP-driven mechanisms of disease in different asthma endotypes. Epithelial alarmins, including TSLP, are released in response to triggers at the epithelium. The alarmins activate multiple innate and adaptive immune responses that participate in overlapping and distinct pathways. TSLP may also mediate structural cell effects that contribute to airway hyperresponsiveness and remodeling. Figure adapted, with permission, from Gauvreau GM et al. Expert Opin Ther Targets. 2020;24(8):777–792.Citation9,Citation74–Citation76
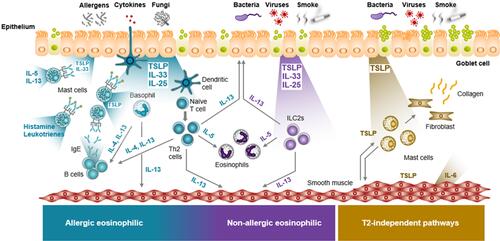
Table 1 Tezepelumab Clinical Trials in Asthma
Figure 2 NAVIGATOR: Annualized Rate of Asthma Exacerbations at Week 52, According to Baseline Biomarker Status.
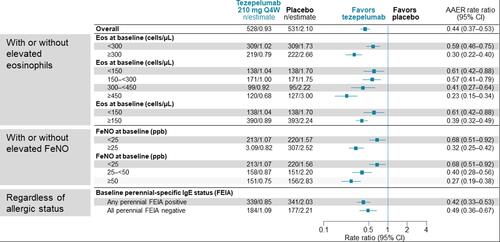
Figure 3 NAVIGATOR: Change from Baseline to Week 52 in Prebronchodilator FEV1.
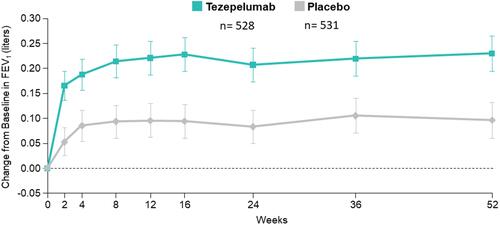
Table 2 Tezepelumab Effect on Patient-Reported Outcomes of Asthma Control and Patient Quality of Life
Table 3 Summary of Adverse Events
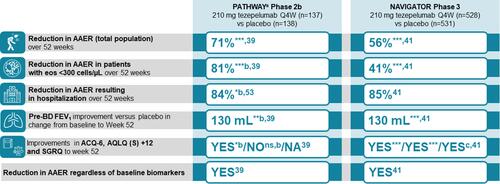
Figure 4 Summary Figure: Tezepelumab Demonstrated Efficacy in a Range of Outcomes Across a Broad Population of Patients.Citation39,Citation41,Citation53 aPATHWAY included three tezepelumab doses; data from the 210-mg dose only are presented; bNominal p value; cScore difference meets criteria for minimal clinically important difference. nsp ≥ 0.05; *p < 0.05; **p < 0.01; ***p < 0.001 compared with placebo group.