Figures & data
Table 1 Baseline Demographic and Clinical Characteristics by Baseline ICS Dose and FEV1% Predicted – ITT Population
Figure 1 Annualized rate of severe exacerbations over the 52-week treatment period in patients with (A) medium-dose ICS and FEV1% predicted ≥60–90% and ≥150 eosinophils/µL, (B) high-dose ICS and FEV1% predicted <60% and ≥150 eosinophils/µL, (C) medium-dose ICS and FEV1% predicted ≥60–90% and ≥150 eosinophils/µL or ≥25 ppb FeNO, and (D) high-dose ICS and FEV1% predicted <60% and ≥150 eosinophils/µL or ≥25 ppb FeNO at baseline – ITT population. ***P<0.001; **P<0.01; *P<0.05 vs matched volume placebo.
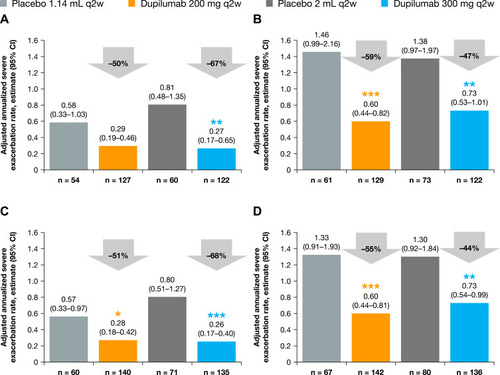
Figure 2 Change from baseline in pre-bronchodilator FEV1 (L) over the 52-week treatment period in patients with (A) medium-dose ICS and FEV1% predicted ≥60–90% and ≥150 eosinophils/µL, (B) high-dose ICS and FEV1% predicted <60% and ≥150 eosinophils/µL, (C) medium-dose ICS and FEV1% predicted ≥60–90% and ≥150 eosinophils/µL or ≥25 ppb FeNO, and (D) high-dose ICS and FEV1% predicted <60% and ≥150 eosinophils/µL or ≥25 ppb FeNO at baseline – ITT population. ***P<0.001; **P<0.01; *P<0.05 vs matched volume placebo.
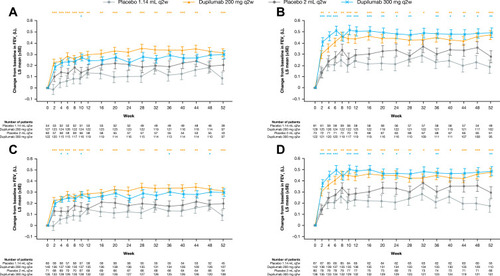
Figure 3 Change from baseline in ACQ-5 score over the 52-week treatment period in patients with (A) medium-dose ICS and FEV1% predicted ≥60–90% and ≥150 eosinophils/µL, (B) high-dose ICS and FEV1% predicted <60% and ≥150 eosinophils/µL, (C) medium-dose ICS and FEV1% predicted ≥60–90% and ≥150 eosinophils/µL or ≥25 ppb FeNO, and (D) high-dose ICS and FEV1% predicted <60% and ≥150 eosinophils/µL or ≥25 ppb FeNO at baseline – ITT population. ***P<0.001; **P<0.01; *P<0.05 vs matched volume placebo.

Figure 4 Change from baseline in AQLQ score over 52 weeks in patients with (A) medium-dose ICS and FEV1% predicted ≥60–90% and ≥150 eosinophils/µL, (B) high-dose ICS and FEV1% predicted <60% and ≥150 eosinophils/µL, (C) medium-dose ICS and FEV1% predicted ≥60–90% and ≥150 eosinophils/µL or ≥25 ppb FeNO, and (D) high-dose ICS and FEV1% predicted <60% and ≥150 eosinophils/µL or ≥25 ppb FeNO at baseline – ITT population. **P<0.01; *P<0.05 vs matched volume placebo.
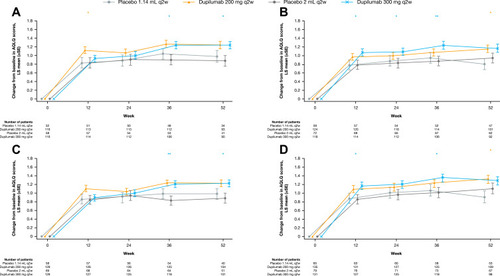
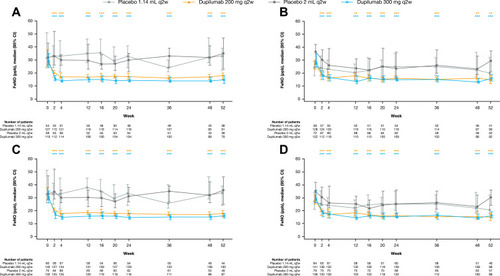
Figure 5 Median FeNO (ppb) during the 52-week treatment period in patients with (A) medium-dose ICS and FEV1% predicted ≥60–90% and ≥150 eosinophils/µL, (B) high-dose ICS and FEV1% predicted <60% and ≥150 eosinophils/µL, (C) medium-dose ICS and FEV1% predicted ≥60–90% and ≥150 eosinophils/µL or ≥25 ppb FeNO, (D) high-dose ICS and FEV1% predicted <60% and ≥150 eosinophils/µL or ≥25 ppb FeNO at baseline – exposed population. ***P<0.001; **P<0.01 vs matched volume placebo (P-values based on change from baseline vs placebo).