Figures & data
Table 1 Agents Shown to Reduce ASM Mass
Figure 1 A cut away view of the structure of a normal and an asthmatic bronchiole, revealing the ASM cell hypertrophy with a thicker cross-sectional area and wider extension of the muscle across the outer surface of the asthmatic bronchiole. The muscle layer itself exhibits increased thickness. The airway lumen of the asthmatic bronchiole is decreased by the edematous swollen pseudo-columnar epithelium and submucosa, increased and edematous extracellular matrix, and the increased ASM thickness. The mucous secretions in the airway are also increased, contributing to the resistance to air flow. Current asthma therapies reduce the tissue swelling and edema, but not the increased thickness of the ASM and ECM.
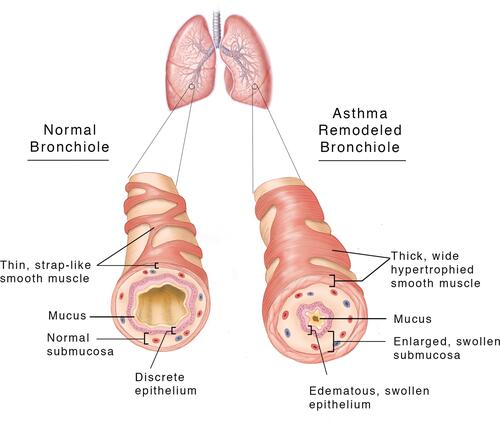
Figure 2 The increased ASM mass in asthma is driven by cell hypertrophy, increased cell migration, and increased cell proliferation. Activation of the airway epithelium by allergens causes the release of EGF, Eotaxin, PDGF, TGF-β, IL-8, and PGD2. Part of the response of these factors is recruitment of inflammatory cells into the airway ECM that release IL-4, IL-5 and IL-13. The factors from the epithelium and the inflammatory cells stimulate myofibroblast migration, proliferation, and hypertrophy, and increased production of ECM components. The epithelial and inflammatory cell-derived factors also stimulate additional production of soluble mediators from the ASM cells that further drive increased ASM mass.
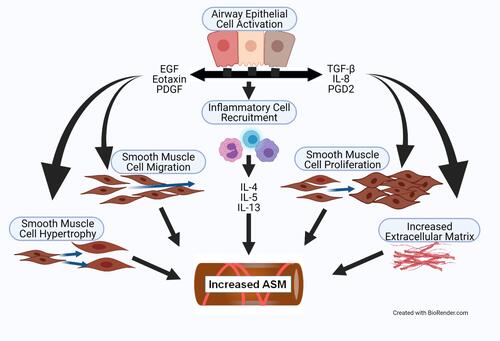
Figure 3 The ASM in chronic asthma exhibits fixed stiffness and muscle spasticity that maintain a baseline increased airway tone with chronic tension. This exerts mechanical compressive forces on the underlying airway epithelium, and also stimulates TGF-β activity via αvβ5 integrin. Chronic inflammation further increases the basal airway tension and epithelial compression. The constant high level of airway spasticity and airway hyperreactivity create a fixed degree of mechanical obstruction.
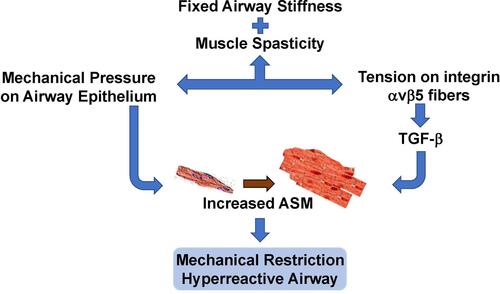
Figure 4 The pathways and factors that create airway stiffness, ASM proliferation, migration, and hypertrophy, and dysregulated autophagy are summarized. Each panel illustrates how different signaling elements and pathways combine to stimulate remodeling of the airway. The autophagy panel illustrates the current dilemma over whether autophagy is increased or decreased in the development of ASM hypertrophy.
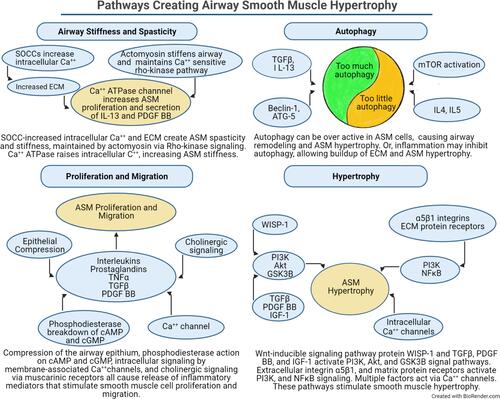
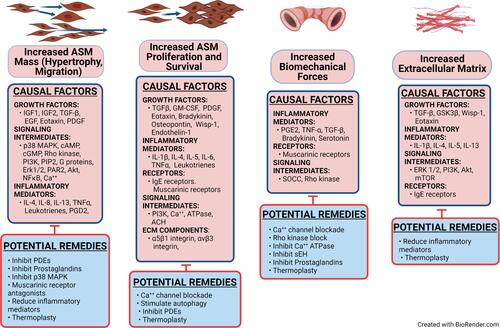
Figure 5 Increased ASM cell hypertrophy and migration, ASM cell proliferation and survival, biomechanical forces, and extracellular matrix are the conditions which can be therapeutically targeted to decrease the physical impact of airway remodeling in asthma. Causal factors are listed separately for each process, along with potential strategies to inhibit these factors. The causal factors include only those for which there is good evidence that they stimulate the specific process. The listed potential remedies include only those shown to antagonize one or more of the causal factors in that column. Some causal factors and potential remedies are listed in more than one column, indicating that some factors are involved in more than one process. This figure should be examined in combination with the , which lists the treatments shown to affect the named process.