Figures & data
Table 1 Identified asthma drugs
Table 2 Selected MedDRA Preferred Terms that reflect arterial thrombotic events
Table 3 Reporting odds ratio and 95% confidence interval data mining algorithm for omalizumab
Figure 1 Study profile.
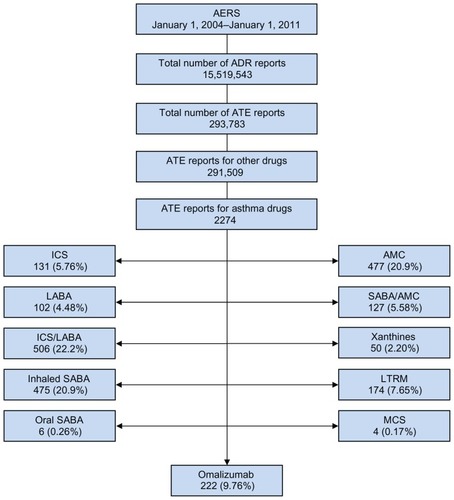
Table 4 Distribution of arterial thrombotic events across exposure groups
Table 5 Distribution of serious arterial thrombotic events across exposure groups
Table 6 Characteristics of arterial thrombotic event reports for omalizumab
Figure 2 Reporting odds ratios and 95% confidence intervals for arterial thrombotic events by asthma medication classes.
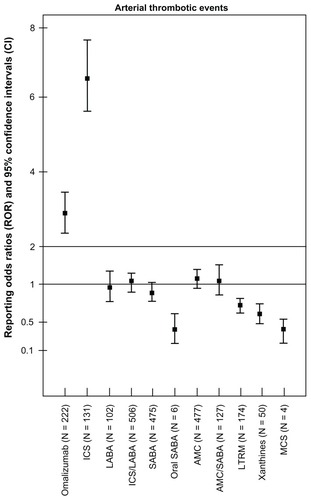
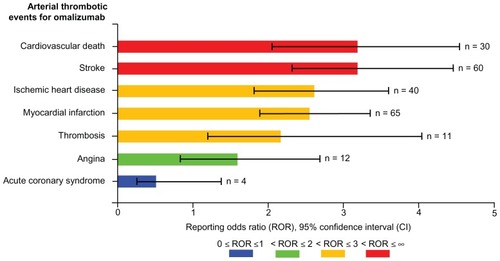
Figure 3 Visual presentation of reporting odds ratios and 95% confidence intervals for selected arterial thrombotic events for omalizumab.