Figures & data
Table 1 Study Treatment Assignment
Figure 1 Study design: 52-week, open-label Phase III safety study. aPatients allocated to receive FF/UMEC/VI 100/62.5/25mcg (selection of FF dose ([100 or 200mcg] depended on patients’ pre-screening therapy [ICS dose/prior use of LAMA] and control status [ACQ-7 total score ≤0.75 or >0.75] []); bPatients switching medication from FF/UMEC/VI 100/62.5/25mcg to 200/62.5/25mcg at Week 24 if their ACQ-7 score was >0.75; this step up was at the investigator’s discretion; cPatients allocated to receive FF/UMEC/VI 200/62.5/25mcg (selection of FF dose [100 or 200mcg] depended on patients’ pre-screening therapy [ICS dose/prior use of LAMA] and control status [ACQ-7 total score ≤0.75 or >0.75] (]).
![Figure 1 Study design: 52-week, open-label Phase III safety study. aPatients allocated to receive FF/UMEC/VI 100/62.5/25mcg (selection of FF dose ([100 or 200mcg] depended on patients’ pre-screening therapy [ICS dose/prior use of LAMA] and control status [ACQ-7 total score ≤0.75 or >0.75] [Table 1]); bPatients switching medication from FF/UMEC/VI 100/62.5/25mcg to 200/62.5/25mcg at Week 24 if their ACQ-7 score was >0.75; this step up was at the investigator’s discretion; cPatients allocated to receive FF/UMEC/VI 200/62.5/25mcg (selection of FF dose [100 or 200mcg] depended on patients’ pre-screening therapy [ICS dose/prior use of LAMA] and control status [ACQ-7 total score ≤0.75 or >0.75] (Table 1]).](/cms/asset/cc637c3d-e95a-4293-a5d5-055a49026c4d/djaa_a_12166899_f0001_c.jpg)
Figure 2 Study disposition. aPatients allocated to receive FF/UMEC/VI 100/62.5/25mcg; bPatients switching medication from FF/UMEC/VI 100/62.5/25mcg to 200/62.5/25mcg at Week 24; cPatients allocated to receive FF/UMEC/VI 200/62.5/25mcg.
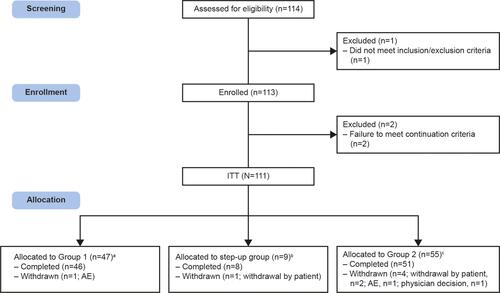
Table 2 Baseline Demographics and Clinical Characteristics (ITT Population)
Table 3 Summary of On-Treatment AEs (ITT Population)
Table 4 Summary of On-Treatment SAEs (ITT Population)
Table 5 Summary of On-Treatment AEs of Special Interest (ITT Population)
