Figures & data
Table 1 INCS Physicochemical, Pharmacokinetic and Pharmacological Characteristics
Figure 1 Relationship between relative glucocorticoid receptor binding affinity and therapeutic daily doses of intranasal corticosteroids (r = 0.833).Citation12,Citation32,Citation34,Citation35,Citation38,Citation62
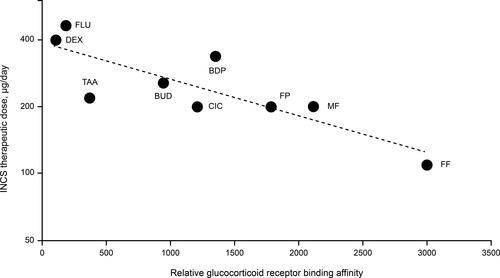
Figure 2 Relationship between relative glucocorticoid receptor binding affinity and human nasal tissue concentration (tissue binding) of intranasal corticosteroids.Citation12,Citation32,Citation34,Citation35,Citation38,Citation62
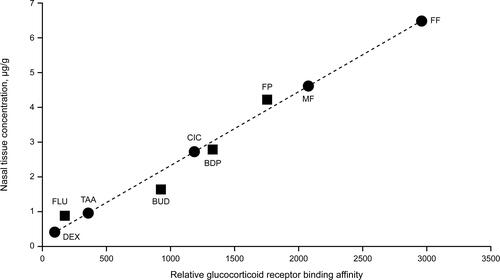
Figure 3 Relationship between relative glucocorticoid receptor binding affinity and the therapeutic index for various intranasal corticosteroids.Citation12,Citation32,Citation34,Citation35,Citation38,Citation62
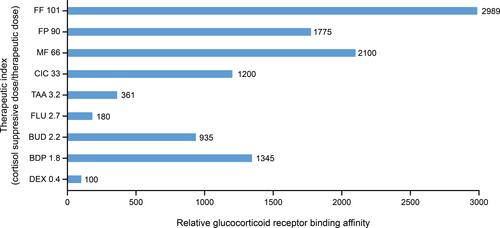
Figure 4 Relationship between systemic bioavailability and the therapeutic index for various intranasal corticosteroids.Citation12,Citation32,Citation34,Citation35,Citation38,Citation62
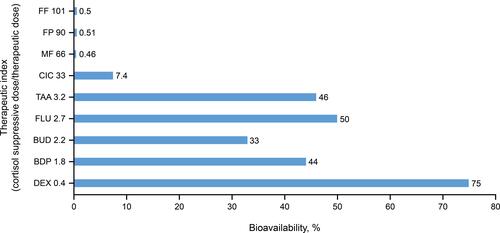
Figure 5 Model-predicted changes in annual growth velocity for a range of doses of intranasal corticosteroids above and below the standard pediatric therapeutic doses. Reprinted from Clin Ther, 26(11), Daley-Yates PT, Richards DH. Relationship between systemic corticosteroid exposure and growth velocity: development and validation of a pharmacokinetic/pharmacodynamic model, 1905–1919, Copyright (2004), with permission from Elsevier.Citation37
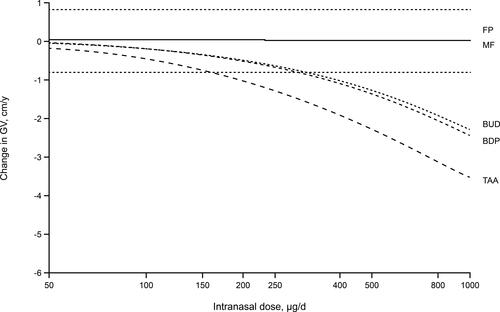
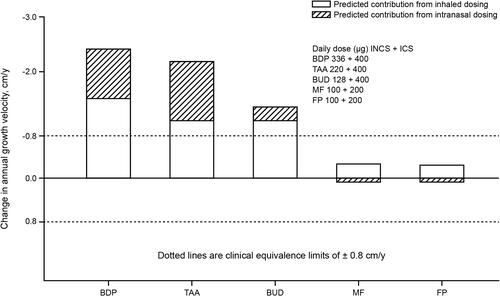
Figure 6 Model-predicted changes in annual growth velocity for combined inhaled and intranasal corticosteroid regimens. Adapted from Clin Ther, 26(11), Daley-Yates PT, Richards DH. Relationship between systemic corticosteroid exposure and growth velocity: development and validation of a pharmacokinetic/pharmacodynamic model, 1905–1919, Copyright (2004), with permission from Elsevier.Citation37