Figures & data
Table 1 Baseline patient features
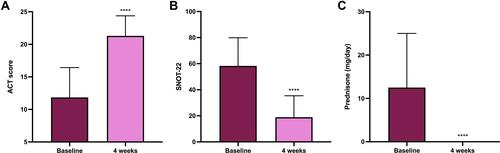
Figure 1 Clinical effects of dupilumab, with regard to ACT score (A), SNOT-22 score (B), and prednisone intake (C). ACT and SNOT-22 values expressed as means ± SD; prednisone intake expressed as medians (IQR). ****p<0.0001.