Figures & data
Figure 1 Epithelial cytokines and other inflammatory mediators and cell types involved in asthma pathogenesis.Citation4,Citation16–19
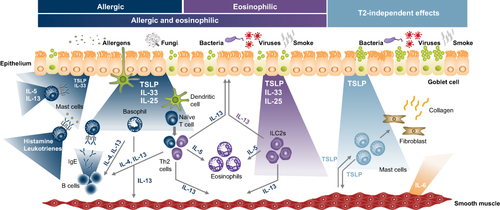
Figure 2 Histopathological staining for TSLP (yellow) in asthmatic bronchiolar epithelial and stromal cells.
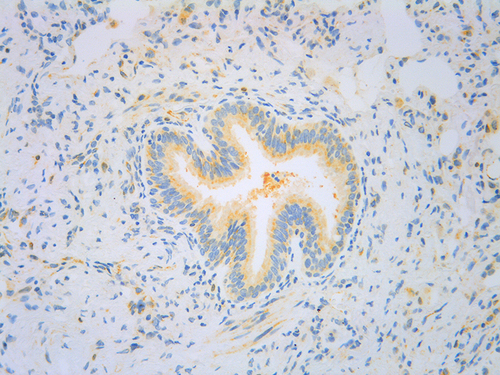
Figure 3 Summary of mechanisms and efficacy of tezepelumab in severe asthma, by asthma subtype.
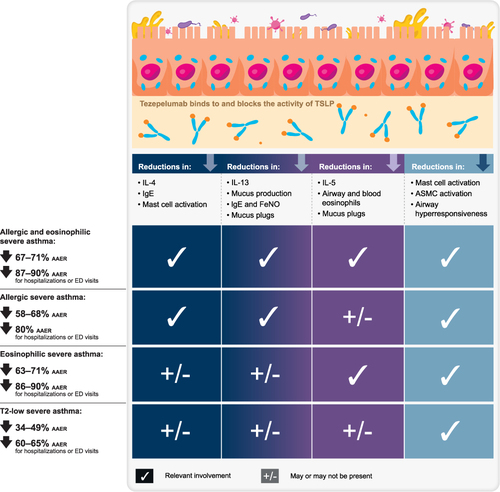
Figure 4 Mean percentage changes from baseline to Week 52 in BEC (A), FeNO levels (B), and total serum IgE levels (C), in patients receiving tezepelumab 210 mg Q4W or placebo, grouped by baseline level of the respective biomarker. A post hoc analysis of the NAVIGATOR Phase 3 study. Adapted from Effect of tezepelumab on asthma inflammatory biomarker levels varies by baseline biomarker levels. J Corren J, J Spahn, C Ambrose, N Martin, G Colice, N Molfino, B Cook. Ann Allergy Asthma Immunol 129(5)(Suppl):S36. Copyright 2022, with permission from Elsevier.Citation38
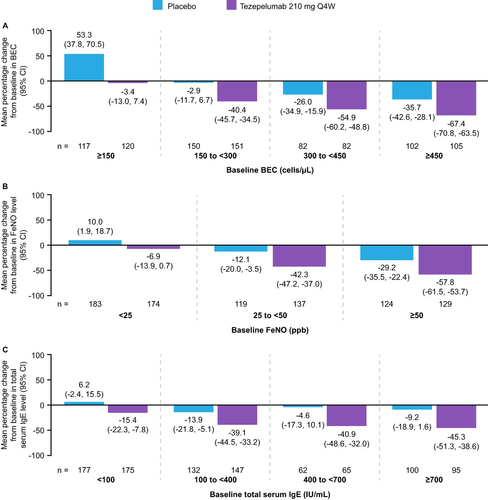
Figure 5 Micrograph images of airway eosinophils (brown staining) from CASCADE, before and after treatment with tezepelumab.
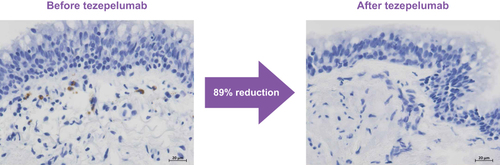
Figure 6 AAER during each season in patients with any seasonal aeroallergen sensitization/allergy overall (A), with baseline BEC <300 cells/μL (B), and with baseline BEC ≥300 cells/μL (C), receiving tezepelumab 210 mg Q4W or placebo. A post hoc analysis of the NAVIGATOR Phase 3 study.
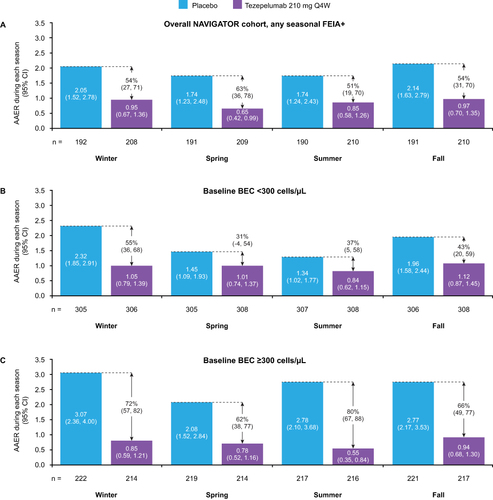
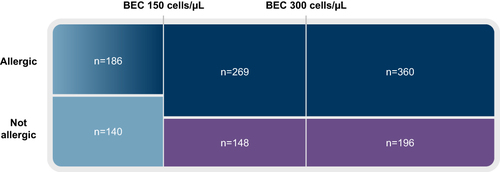
Figure 7 Proportional representation of patients by baseline BEC and allergy to perennial aeroallergens in the pooled PATHWAY and NAVIGATOR populations.