Figures & data
Table 1 Patient Information, Allergy Symptoms, Total IgE Values (kU/L) and SPT Results
Figure 1 The survey of locust allergy in a cohort of 57 subjects. (A) Number of subjects sensitized to locust (males, females and total). (B) Suspected allergic disorders to locust in subjects (males, females and total). (C) Effect of other allergic histories on occupational allergies to locusts. (D) ROC curve of interval exposure and continuous exposure. The blue line was interval exposure indicating occupational exposure lasting for 2–3 h per day. The red line was continuous exposure indicating occupational exposure continuing for one and half year.
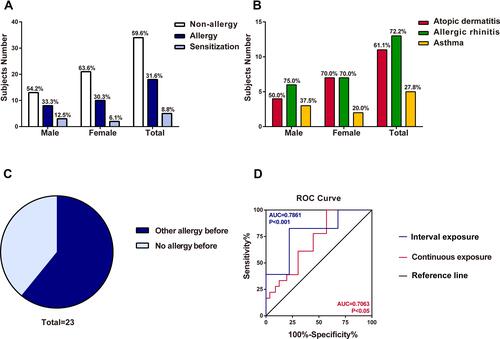
Figure 2 Identification of the sensitization to locust proteins of the subjects. (A) Measurement of sIgE for locust proteins by ELISA. Statistically significant differences are shown using different letters above the bars (P < 0.05, one-way ANOVA), a is significant different from b, ab is not different from a or b. (B and C) sIgE immunoblotting of locust protein. (B) Lane 1, protein marker. Lane 2, SDS-PAGE of locust extracts protein. Lane 3, immunoblotting with sera pool from 10 locust-allergic subjects. Lane 4, immunoblotting with sera pool from 10 control subjects. (C) Lane 1, protein marker. Lane 2, SDS-PAGE of locust extracts protein. Lanes 3–11, immunoblotting with individual serum from locust-allergic subjects. Lane 12, pooled sera from 10 control subjects.
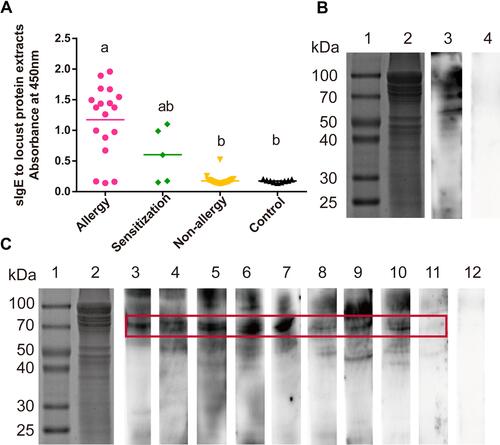
Figure 3 Mass analysis, peptide matches, purification and conserved domains analysis of hexamerin-2 protein. (A) Mature protein sequence, peptides verified by the mass spectrum fingerprint are shown in red and highlighted (NCBI accession ACU78069.1). (B) SDS-PAGE of the purified hexamerin-2 protein. Lane 1, protein marker. Lane 2, SDS-PAGE of the hexamerin-2 protein. (C) Three domains of the hexamerin-2 protein were analysed in the NCBI database: hemocyanin-N domain, hemocyanin-M domain, hemocyanin-C domain.
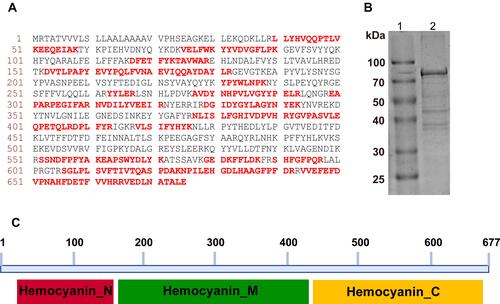
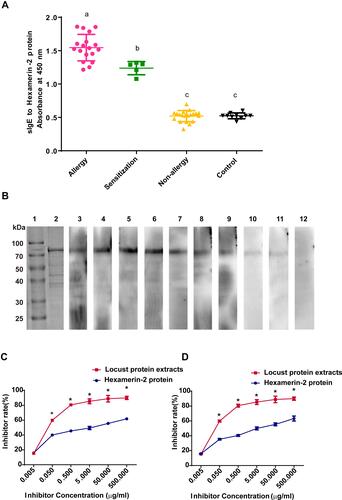
Figure 4 Identification of the sensitization to the hexamerin-2 protein of the subjects. (A) Measurement of sIgE for the hexamerin-2 protein by ELISA. Statistically significant differences are shown using different letters (ie, a, b, c) above the bars (P < 0.05, one-way ANOVA). (B) sIgE immunoblotting of Hexamerin-2 protein. Lane 1, protein marker. Lane 2, SDS-PAGE of the hexamerin-2 protein. Lanes 3–11, Immunoblotting with individual serum from 9 locust-allergic subjects. Lane 12, immunoblotting with sera pool from 10 control subjects. (C and D) Examination of serum IgE reactivity to the hexamerin-2 protein by competitive ELISA, representing the serum from locust-allergic patients P2 (C) and P3 (D), respectively. P values < 0.05 were considered significant.