Figures & data
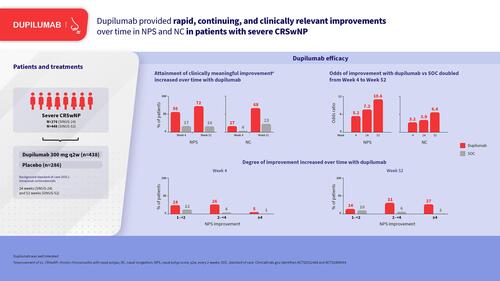
Figure 1 Proportion of patients with NPS and NC score change (improved, stable, and worsened) over time (Weeks 4 through 52) in SINUS-52.
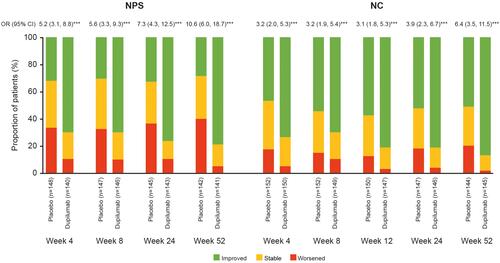
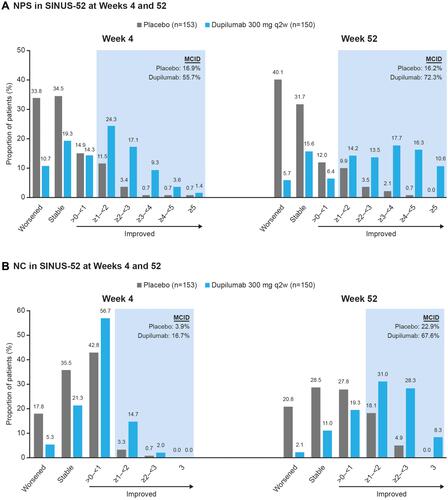
Figure 2 Proportion of patients with (A) NPS and (B) NC score change (stable, worsened, and categories of improvement) at Weeks 4 and 52 in SINUS-52.