Figures & data
Table 1 Breakdown of the Criteria for Clinical Features of Obstructive Lung Disease
Table 2 Demographic and Baseline Characteristics of Subgroups of Patients with CRSwNP from SINUS-24 and SINUS-52 with a Diagnosis of Asthma or with Clinical Features of Obstructive Lung Disease
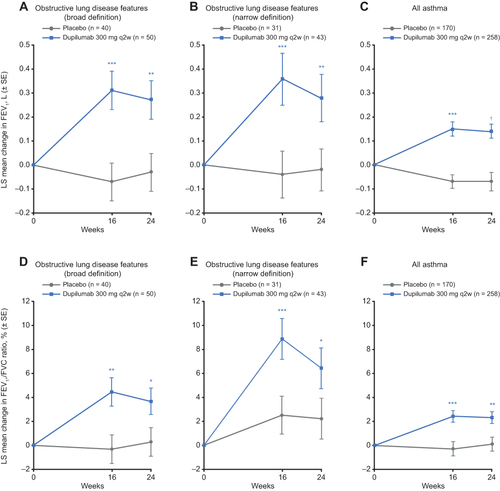
Figure 1 Effect of dupilumab on NPS in patients with CRSwNP and clinical features of obstructive lung disease.
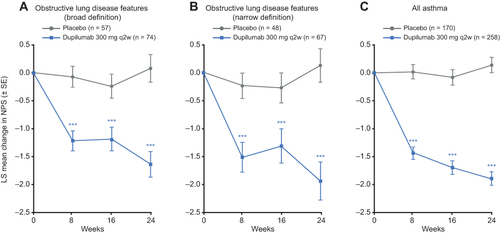
Figure 2 Effect of dupilumab on UPSIT score in patients with CRSwNP and clinical features of obstructive lung disease.
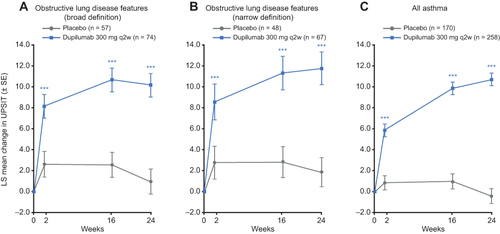
Figure 3 Effect of dupilumab on SNOT-22 total score in patients with CRSwNP and clinical features of obstructive lung disease.
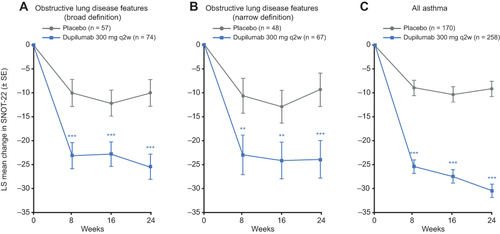
Figure 4 Effect of dupilumab on lung function in patients with CRSwNP and clinical features of obstructive lung disease.