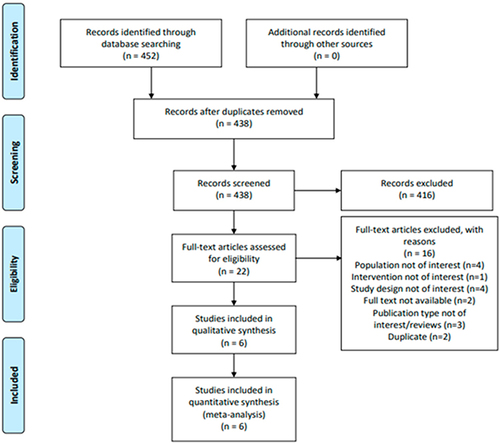
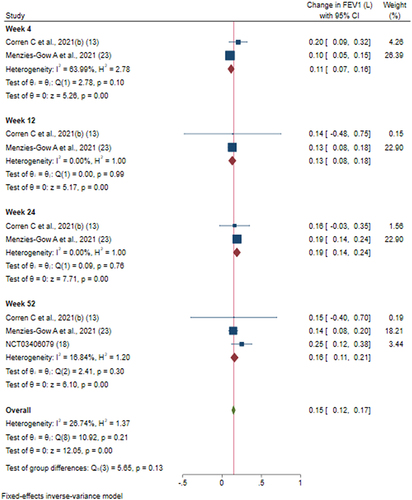
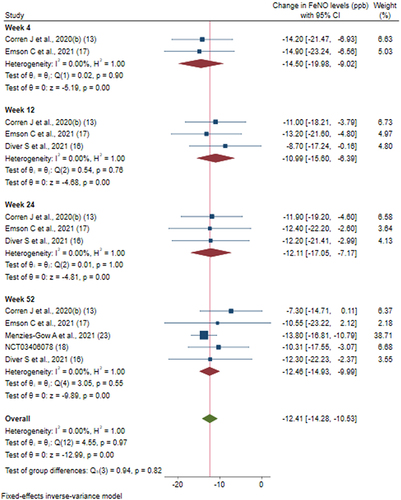
Figures & data
Table 1 Study Characteristics of Included Studies
Figure 2 Assessment of the risk of bias in included studies with Cochrane domain-based quality assessment tool.
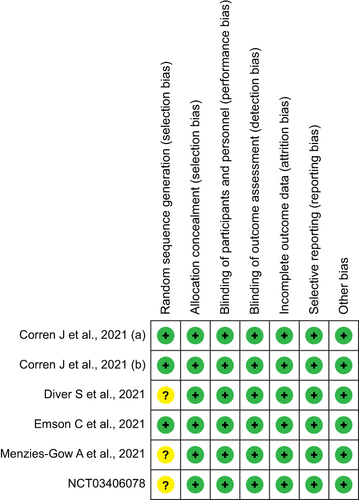
Figure 4 Efficacy of tezepelumab versus placebo on rate of asthma exacerbation based on different doses of intervention.
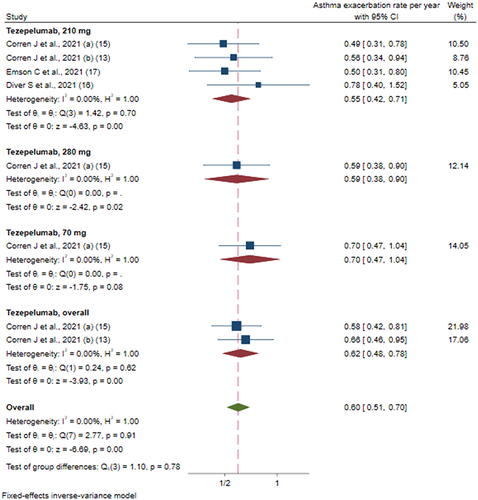
Figure 5 Efficacy of tezepelumab versus placebo on asthma related quality of life score based on different doses of intervention; Tezepelumab, overall: studies which reported data for combining of all the dosages (70 mg + 210 mg + 280 mg).
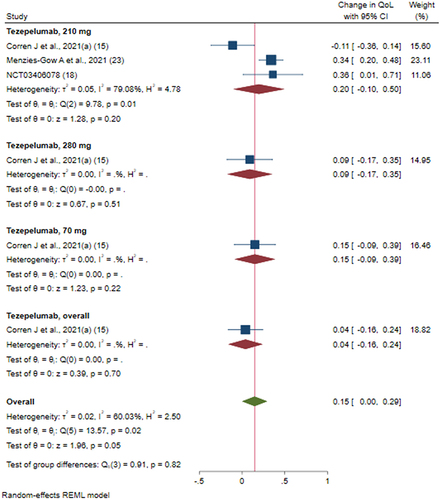
Figure 6 Efficacy of tezepelumab versus placebo on blood eosinophil count based on duration of intervention.
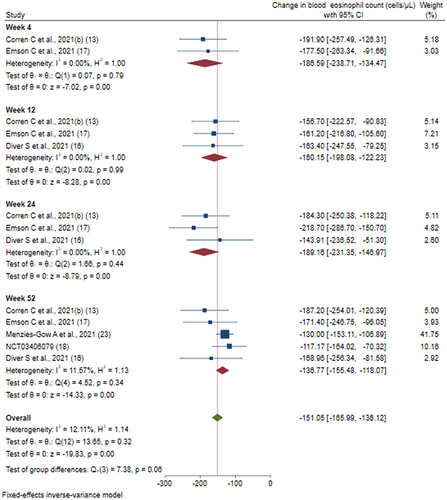
Figure 8 Efficacy of tezepelumab versus placebo on total serum IgE levels based on duration of intervention.
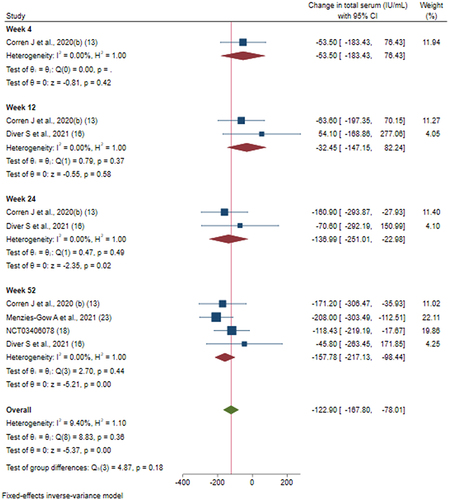
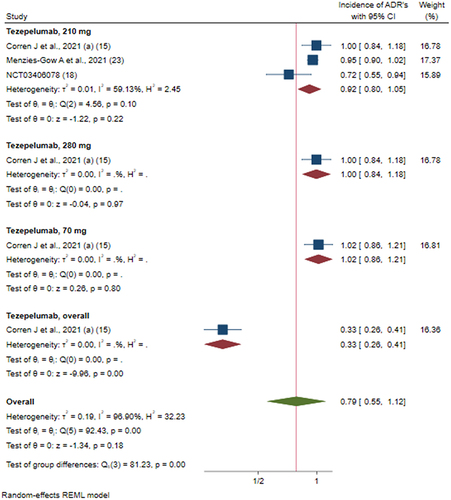
Figure 9 Efficacy of tezepelumab versus placebo on incidence of ADR’s based on different doses of intervention; Tezepelumab, overall: studies which reported data for combining of all the dosages (70 mg + 210 mg + 280 mg).