Figures & data
Figure 1 Production of bradykinin through kallikrein-dependent reactions. Reprinted from J Allergy Clin Immunol, Vol 126, no 5, Kaplan AP, Enzymatic pathways in the pathogenesis of hereditary angioedema: the role of C1 inhibitor therapy, pp918-925, Copyright 2023, with permission from Elsevier.Citation17
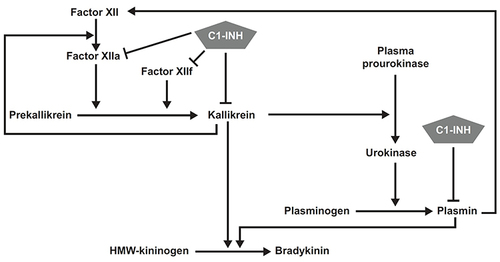
Table 1 Study Design Elements in Randomized Controlled Trials of Long-Term Prophylaxis with C1-INH Replacement Therapies in Adult Patients with HAE
Table 2 Primary Efficacy and Safety Outcomes in Randomized Controlled Trials of Long-Term HAE Prophylaxis with C1-INH Replacement Therapy
Table 3 Additional Efficacy Endpoints in Randomized Controlled Trials of Long-Term HAE Prophylaxis with C1-INH Replacement Therapy
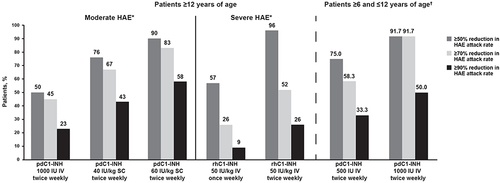
Figure 2 Mean percentage reductions in acute hereditary angioedema attack rates with C1-INH replacement therapies, by dose.