Figures & data
Figure 1 Mutations in genes linked to some forms of HAE-nI-C1-INH and their roles within the fibrinolytic and kallikrein/kinin systems.Citation20,Citation21,Citation24,Citation25 Adapted from Bork K, Machnig T, Wulff K, Witzke G, Prusty S, Hardt J. Clinical features of genetically characterized types of hereditary angioedema with normal C1 inhibitor: a systematic review of qualitative evidence. Orphanet J Rare Dis. 2020;15(1):289. Creative Commons.Citation21
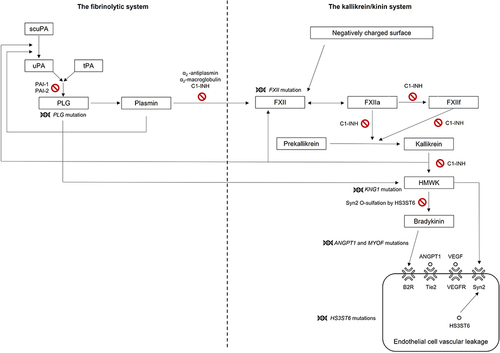
Table 1 Summary of Genes That Have Been Identified to Contain Mutations in Patients with HAE-nI-C1-INH and the Proposed Roles of Such Mutations in Angioedema
Figure 2 Diagnostic algorithms for (a) HAE-C1-INH and (b) HAE-nI-C1-INH, based on the known clinical and genetic characteristics of the diseases. Presence of the supportive evidence shown in the boxes with blue outlines is not required for a confirmed diagnosis of HAE.Citation1,Citation45,Citation46
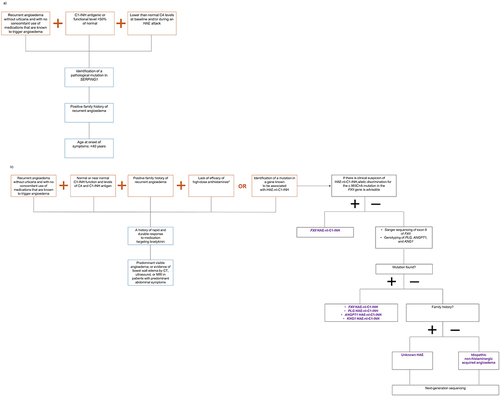
Table 2 Proposed Mode of Action of Treatments Offered to Patients with HAE-nI-C1-INH
