Figures & data
Table 1 Cohort 1 (N=131): Clinical Characteristics and Maintenance Medication in the Past at Entry into the GAN Registry, ie, Before Starting Mepolizumab Therapy
Table 2 Cohort 1: Clinical Characteristics in the Subset of Patients (N=87) with Available 4-Month Follow-Up Data After Initiating Mepolizumab Therapy
Table 3 Cohort 2 (N=220): Asthma Symptoms, Other Allergic Conditions/Comorbidities, and Rescue Medication at Entry into the GAN Registry
Figure 1 Cohort 2: Percentage of patients with symptoms (a) during the day or (b) during the night in the subset of patients with available 1-year follow-up data after entry into the GAN registry (N=80 and N=79 with available data).
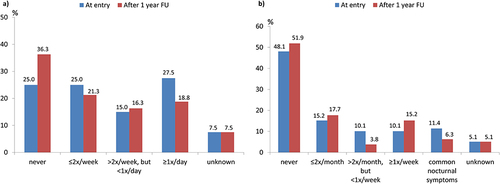
Table 4 Cohort 2: Lung Function and Symptom Control in the Subset of Patients (N=89) with Available 1-Year Follow-Up Data After Entry into the GAN Registry
