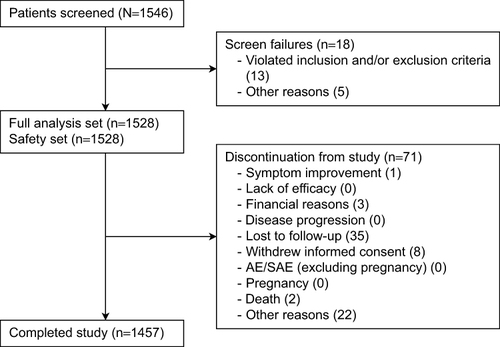
Figures & data
Table 1 Patient Demographics and Clinical Characteristics at Baseline
Table 2 Overview of Adverse Events (Safety Analysis Set)
Figure 2 Effectiveness endpoints in the overall population. (A) GETE responder. GETE of excellent or good was defined as the responder; GETE of moderate, poor, or worsening was defined as the non-responder. The percentages are calculated based on the number of patients who had been evaluated by the investigator and patient in each visit. (B) Mini AQLQ mean change from baseline. (C) PAQLQ change from baseline (patient <18 years). (D) Asthma exacerbation events. The summary of the annualized number of asthma exacerbation events that occurred at 0–16 weeks and 0–24 weeks.
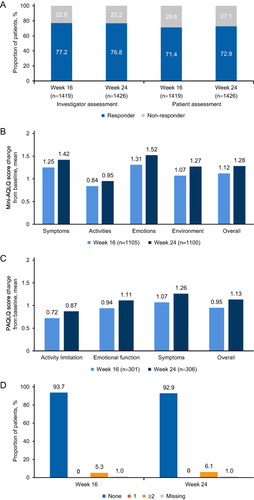
Table 3 Summary of Pulmonary Function Test