Figures & data
Figure 1 Observed ICS exposure and projected additional as-needed ICS exposure if each SABA inhalation contained 80 μg budesonide, relative to maximum FDA-approved doses, by maintenance group.
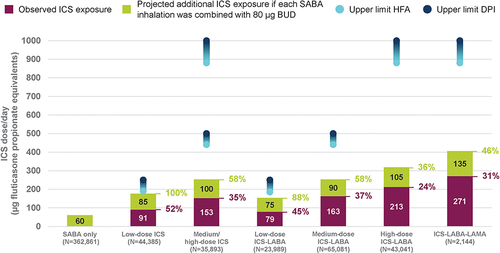
Table 1 Estimated Total ICS Exposure from Maintenance Plus As-Needed SABA-ICS Relative to Observed SCS Exposure
