Figures & data
Figure 1 Patient disposition. aThe four inclusion criteria were prescription of high-dose inhaled corticosteroids or inhaled corticosteroids/long-acting β2-agonists during the baseline period of 24 weeks before the index date; a record of ≥1 hospital visit during the baseline period, ≥2 hospital visits during the observation period, and ≥1 hospital visit after the observation period; and a total maintenance oral corticosteroid prescription duration ≥12 weeks during the baseline period (including the observation start date); and age ≥16 years at the start of the observation period. bPatients with the following selected autoimmune diseases were excluded: rheumatoid arthritis with rheumatoid factor (ICD-10 code: M05), other rheumatoid arthritis (M06), polyarteritis nodosa and related conditions (M30), lupus erythematosus (L93), systemic lupus erythematosus (M32), Crohn disease (regional enteritis) (K50), ulcerative colitis (K51), other noninfective gastroenteritis and colitis (K52), and nephrotic syndrome with minor glomerular abnormality (N04).
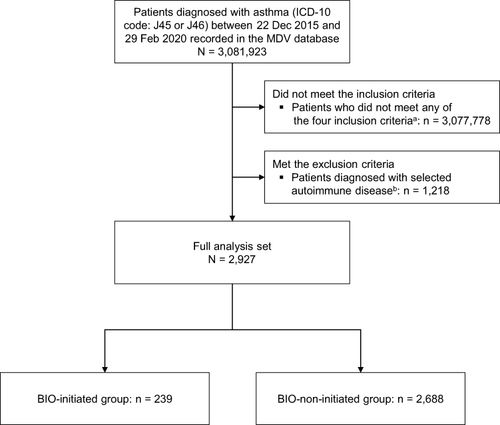
Table 1 Background Characteristics of Patients
Table 2 Reduction in Daily Maintenance OCS Dose at Week 24
Table 3 Proportion of Patients Who Achieved a >0%, ≥25%, ≥50%, and 100% Reduction in Their Daily Oral Corticosteroid Maintenance Dose at Week 24
