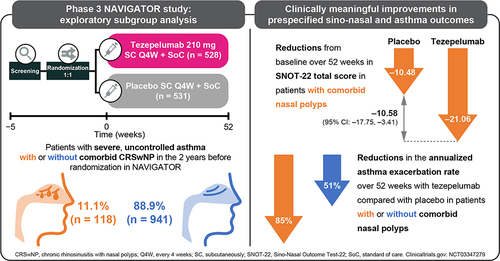
Figures & data
Table 1 Baseline Demographics and Clinical Characteristics of Patients with and without Nasal Polyps in the 2 Years Before Randomization in the NAVIGATOR Study
Figure 1 AAER over 52 weeks in patients with or without nasal polyps in the 2 years before randomization in the NAVIGATOR study.
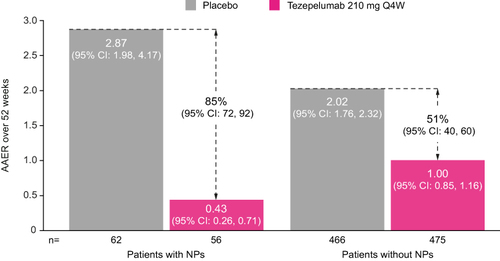
Table 2 Change from Baseline to Week 52 in Pre-Bronchodilator FEV1 and ACQ-6, AQLQ(S)+12 and ASD Scores in Patients with and without Nasal Polyps in the 2 Years Before Randomization in the NAVIGATOR Study
Figure 2 Change from baseline in SNOT-22 total score over 52 weeks in patients with nasal polyps in the 2 years before randomization in the NAVIGATOR study.
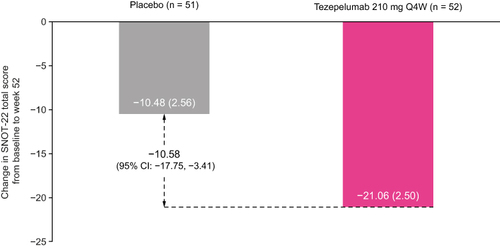
Figure 3 Change from baseline in SNOT-22 nasal (A), ear/facial (B), sleep (C), function (D) and emotion (E) domain scores over 52 weeks in patients with nasal polyps in the 2 years before randomization in the NAVIGATOR study.
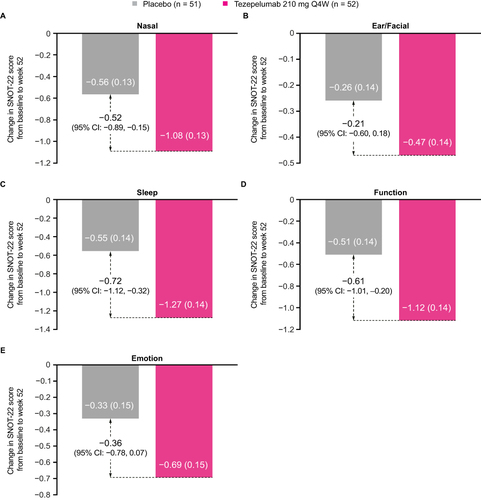
Table 3 Baseline SNOT-22 Total and Domain Scores in Patients with Nasal Polyps in the 2 Years Before Randomization in the NAVIGATOR Study
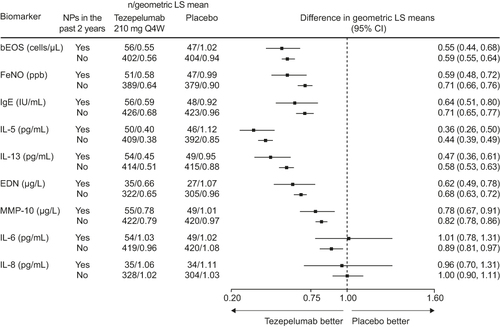
Figure 4 Change from baseline in exploratory biomarkers over 52 weeks with tezepelumab compared with placebo in patients with or without NPs in the 2 years before randomization in the NAVIGATOR study.