Figures & data
Table 1 Characteristics of the of All Participants (Control and Asthma Groups)
Table 2 Clinical Characteristics of Asthma Patients (n = 141)
Table 3 Differences Between Asthmatic and Control in Studied Biomarkers
Table 4 Differences Between Asthmatic Groups According to ACT Score in Studied Biomarkers
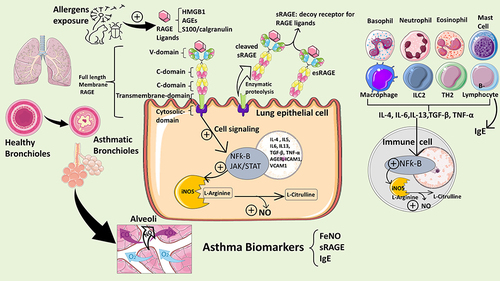
Figure 1 Box plots demonstrating comparisons between asthmatic children who take inhaled corticosteroids (ICS) and who do not (A–D), and between asthmatic children who take systemic steroids and who do not (E–H) in terms of ACT score, FeNO, sRAGE and esRAGE levels. ACT score was significantly different between children who are under ICS and who are not (chi square = 8.705, p = 0.003, df = 1) as in (A). However, asthmatic children who were treated with systemic steroids had significantly lower ACT scores (chi square = 11.813, p = 0.001, df = 1) as in (E). On the other hand, FeNO level, sRAGE, or esRAGE were not significantly affected by ICS or systemic steroids (chi square = 3.71, p = 0.39, df = 6) as in (B–D and F–H; consequently); *P value of ≤.05 is considered significant.
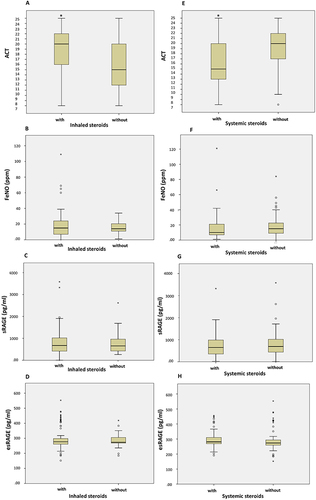
Figure 2 Comparisons between asthmatic children who have concomitant allergic disease and who have not in levels of FeNO, sRAGE and esRAGE/RAGE ratio. (A) asthmatic children without and with allergic rhinitis; ratio of esRAGE/sRAGE, was significantly lower compared to children who have not; (0.92 ± 2.08 vs. 1.57 ± 8.59, Asymp. Sig. (2-tailed) =0.026) and a trend for the sRAGE level to be lower, while FeNO levels were not different; (B) asthmatic children without and with atopic dermatitis; atopic dermatitis was associated with higher FeNO levels (22.58 ± 16.49 vs. 16.80 ± 15.86, Asymp. Sig. (2-tailed) =0 0.014); (C) asthmatic children without and with food allergy; food allergy was associated with higher sRAGE levels; (982.23 ± 480.79 vs. 742.96± 563.93, Asymp. Sig. (2-tailed) = 0.017) and a trend to reduced esRAGE/RAGE ratio. *P value of ≤.05 is considered significant.
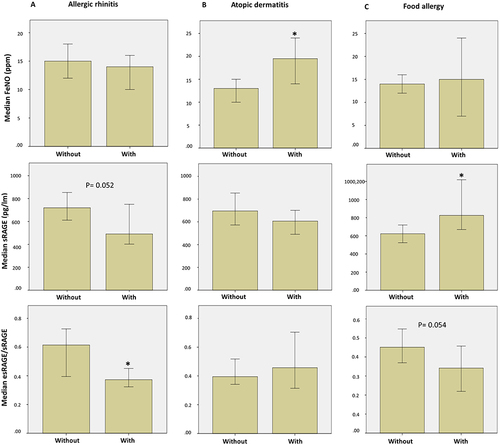
Table 5 Factors Affecting FENO Level Among All Participants, Control Group and Asthmatic Patients’
Table 6 Factors Affecting ESRAGE/SRAGE Among All Participants, Control Group and Asthmatic Patients
Figure 3 ROC curve of FeNO, sRAGE, esRAGE, esRAGE/RAGE, among different study groups. (A) ROC curve of FeNO, sRAGE, esRAGE, esRAGE/RAGE among all participants (n = 258). AUC of test variables in identifying asthma (GINA standards) are depicted in the tables under the corresponding curve; number of actual positive results(asthmatics) = 140 (B) ROC curve of FeNO, sRAGE, esRAGE, esRAGE/RAGE in asthmatic patients (n = 140). AUC of test variables in identifying lack of control (ACT as gold standard) are depicted in the tables under the corresponding curve; number of actual positive results (uncontrolled asthma) = 43. a, Under the nonparametric assumption; b, Null hypothesis: true area = 0.5; *P value of ≤.05 is considered significant.
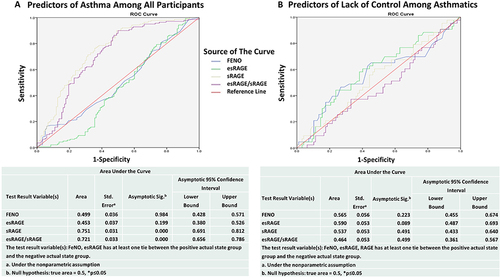
Figure 4 This schematic summarizes the mechanisms underlying allergic airway inflammation in asthma involving RAGE signaling and biosynthesis of nitric oxide (NO). Allergens trigger release of RAGE ligands such as HMGB1, S100/calgranulins which bind to and activate RAGE on lung epithelial cells. RAGE structure is depicted showing its variable domain (V-domain), two constant domains(C-domain), and cytosolic domain. The RAGE protein exists as full-length membrane-bound RAGE (mRAGE), as well as a soluble form (sRAGE), which lacks the transmembrane and signaling domains, and functions as a decoy receptor. sRAGE can be produced endogenously by alternative splicing (esRAGE) or through proteolytic cleavage of the full-length mRAGE. Upon ligand binding, intracellular signaling cascades are initiated which leads to transcriptional activation of NF-κB- and STAT-dependent gene transcription. RAGE-dependent activation of NF-κB induces a positive feedback loop by inducing RAGE (AGER gene) and NF-kB gene transcription and is suspected to stimulate release of cytokines that activate resident immune cells in the lung and is also released into the circulation to activate TH2 cells and ILC2s producing large amounts of IL-4, IL-5 and IL-13 and other cytokines activating B cells to produce allergy-specific globulins (IgE). The IgE specific to allergens will then bind to eosinophils to exacerbate allergic airway inflammation and airway hyperresponsiveness. The biosynthesis of NO in the airways is induced by inducible NO Synthase (iNOS) expressed in lung epithelial cells and inflammatory cells (basophils, eosinophils, neutrophils, mast cells, B or T lymphocytes). NO produced intracellularly will diffuse to the lumen of the airways. Upregulation of iNOS induced by NF-kB during allergic inflammation generates elevated level of exhaled NO in the bronchial airways which can be detected as “FeNO” indicating degree of inflammation.