Figures & data
Table 1 Baseline Demographic and Disease Characteristics of Children with Moderate-to-Severe Asthma Who Did or Did Not Achieve 5% and 10% Improvements in Pre-Bronchodilator ppFEV1 from Baseline to Week 12
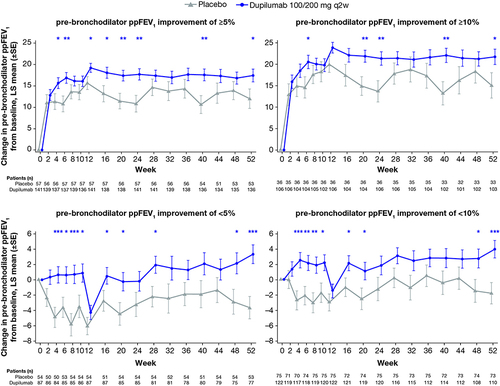
Figure 1 Reduction in severe asthma exacerbation rates during the VOYAGE Week 12 to 52 treatment period in children with moderate-to-severe asthma who did or did not achieve 5% and 10% improvements in pre-bronchodilator ppFEV1 from baseline by Week 12.
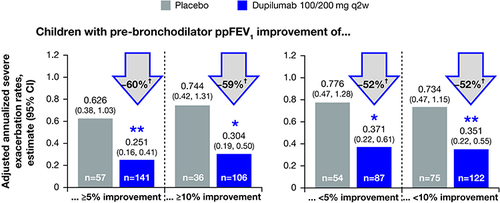
Figure 2 Change of pre-bronchodilator ppFEV1 over time in children with moderate-to-severe asthma who did or did not achieve 5% and 10% improvements in pre-bronchodilator ppFEV1 from baseline by Week 12.