Figures & data
Table 1 Baseline (12-Month Pre-Index Period) Demographics and Characteristics
Table 2 Baseline (12-Month Pre-Index Period) Disease-Related Comorbidities Reported in ≥1% of Patients
Figure 1 Mean change in total NPS from baseline following benralizumab initiation. Changes from baseline were evaluated in the subset of patients who had both baseline and follow-up available data. *All data. Baseline (n=91) 3.8 (2.4); >0–<3 months, n=20; ≥3–<6 months, n=22; ≥6–<9 months, n=21; ≥9–≤12 months, n=35; up to 12 months, n=63.
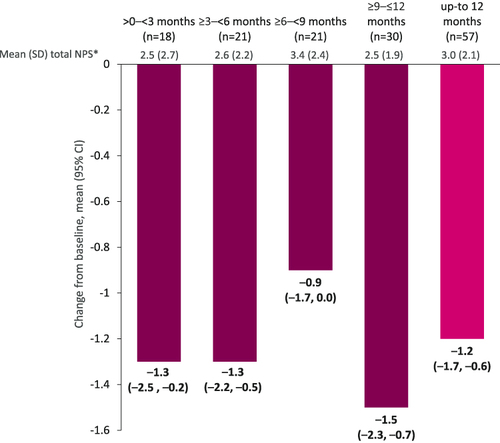
Figure 2 Percentage of patients with clinically meaningful improvement in (A) NPS, and (B) SNOT-22 total score, following benralizumab initiation. Improvement was defined as total NPS reduction ≥1 point; SNOT-22 total score reduction ≥8.9 points. Within the first 6 months, 54.8% (17/31) of patients achieved clinically meaningful reduction in NPS and 48.8% (21/43) in SNOT-22 score.
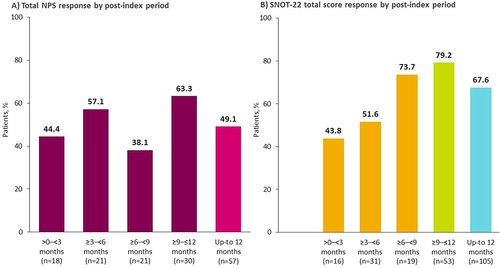
Figure 3 Mean change in SNOT-22 total score from baseline following benralizumab initiation. *All data. Baseline (n=161) 47.5 (22.6); >0–<3 months, n=18; ≥3–<6 months, n=34; ≥6–<9 months, n=22; ≥9–≤12 months, n=54; up to 12 months, n=114.
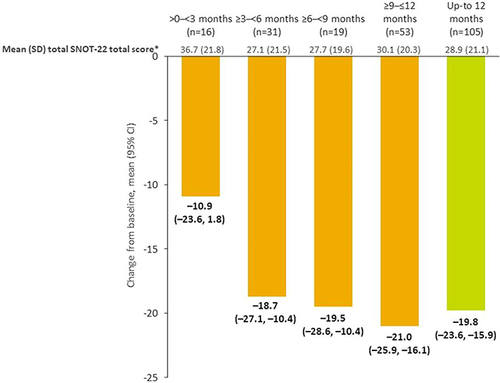
Figure 4 Asthma outcomes at baseline (12-month pre-index period) and following benralizumab initiation: (A) AER, (B) patients with exacerbations, (C) mean ACQ-6 score, and (D) mean ACT score. Changes from baseline were evaluated in the subset of patients who had both baseline and follow-up available data.
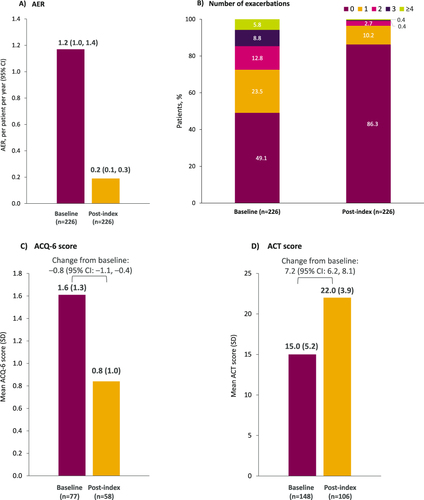
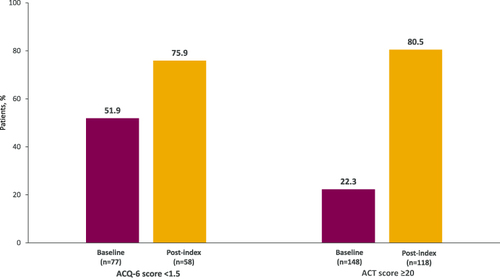
Figure 5 Percentage of patients with controlled asthma at baseline (12-month pre-index period) and during follow-up. Controlled asthma defined as ACQ-6 score <1.5 (well-controlled/partly controlled) or ACT score ≥20 (well-controlled).