Figures & data
Table 1 Baseline Demographics and Clinical Characteristics
Figure 2 Proportion of patients with select comorbidities at baseline. p-values that reached statistical significance are indicated in bold.
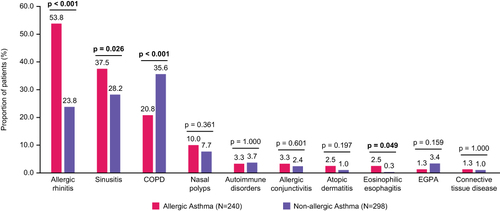
Figure 3 Rate of asthma exacerbations: (A) Proportion of patients with ≥1 asthma exacerbation during baseline and follow-up; (B) Change in mean count of asthma exacerbations between baseline and follow-up. p-values that reached statistical significance are indicated in bold.
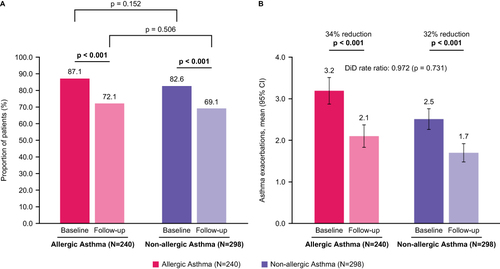
Table 2 OCS Daily Dose During Baseline and Follow-Up Among All Patients
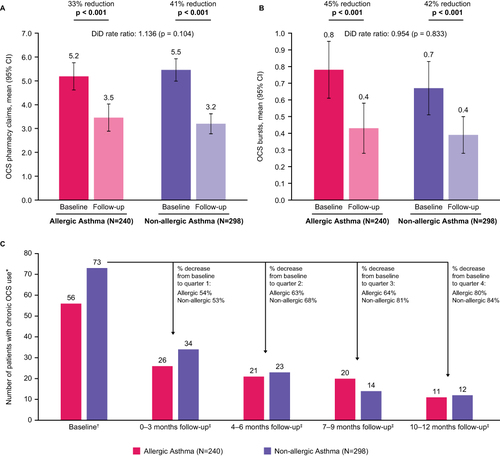
Figure 4 OCS use: (A) Mean count of OCS pharmacy claims; (B) Mean count of OCS bursts; (C) Number of patients with chronic OCS use. p-values that reached statistical significance are indicated in bold. *Pre-index counts (ie from Baseline period) are among all allergic (N=240) and non-allergic (N=298) patients; post-index counts (ie from within the 12-month follow-up period) are among patients with chronic OCS use (≥10 mg/day in the 90 days pre-index); †Measured during the 90 days period prior to the index date; ‡Measured during the 12-month follow-up period among patients with chronic OCS use in the 90 days period prior to the index date.