Figures & data
Table 1 Characteristics for Patients Who Were Eligible, Enrolled, and Initiated Biologics After Enrollment
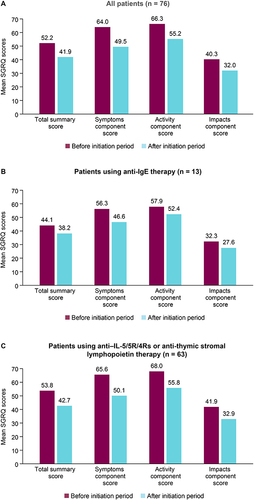
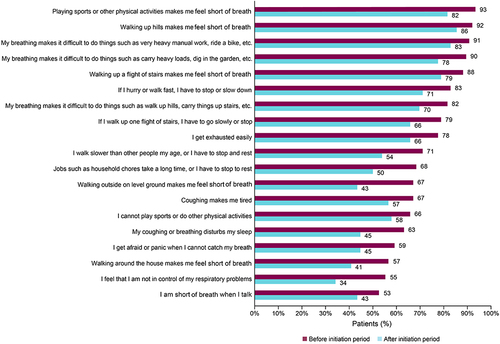
Figure 1 SGRQ scores 6 months before and 12 to 18 months after initiation of biologics. (A) All patients (n = 76). (B) Patients using anti-IgE therapy (n = 13). (C) Patients using anti–IL-5/5R/4Rs or anti-thymic stromal lymphopoietin therapy (n = 63).