Figures & data
Table 1 Reasons for Starting Benralizumab in Group A (Maintenance Therapy Phase)
Table 2 Baseline Characteristics of Patients Starting Benralizumab in in Group A and B (Maintenance and Induction Therapy Phase)
Table 3 Comparison of Outcome Parameters at Baseline (Start) and After 12 Months of Benralizumab Therapy in Group A (Maintenance Therapy Phase)
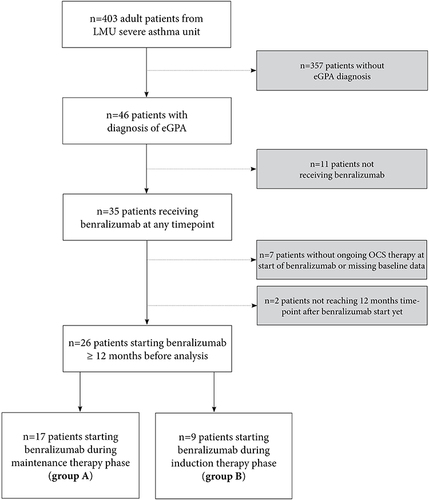
Figure 2 Comparison of parameters of OCS use and symptoms (A), lung function (B), and biomarkers (C) before and after 12 months of benralizumab of patients in maintenance cohort. In this analysis we included all 15 patients who continued benralizumab for 12 months. Statistics: (A), (C): Wilcoxon matched-pairs signed-rank test; (B): paired t-test, *p<0.05, **p< 0.01, ***p<0.001.
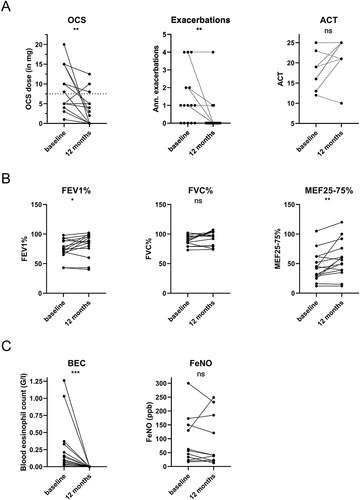
Table 4 Comparison of Outcome Parameters at Baseline (Start of Benralizumab) and After 12 Months of Benralizumab Therapy in Group B (Induction Therapy Phase)
Figure 3 Comparison of parameters of OCS use and exacerbations (A), lung function (B), and biomarkers (C) before and after 12 months of benralizumab in induction cohort. In this analysis we included all 7 patients who continued benralizumab for 12 months. Statistics: Wilcoxon matched-pairs signed-rank test, *p<0.05.
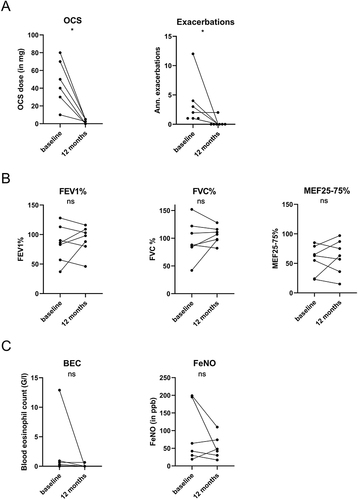
Table 5 Long-Term Follow-Up in Patients Who Continued Benralizumab Beyond 12-Month Timepoint