Figures & data
Figure 1 Patient selection process and study design. Inclusion criteria: (A) at least one prescription for tiotropium, (B) recorded diagnosis of asthma, (C) no recorded diagnosis of COPD, (D) at least 18 years of age at first tiotropium prescription, and (E) full 12 months of data before (baseline) and after (outcome) first tiotropium prescription.
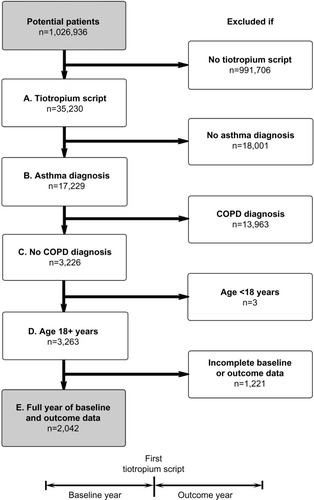
Table 1 Effectiveness measures compared before and after addition of tiotropium
Table 2 Key descriptive characteristics at baseline
Table 3 Comparison of effectiveness measures before (baseline) and after (outcome) addition of tiotropium
Figure 2 Exacerbation rates before (baseline) and after (outcome) addition of tiotropium. P<0.001 (marginal homogeneity test) comparing baseline and outcome years.
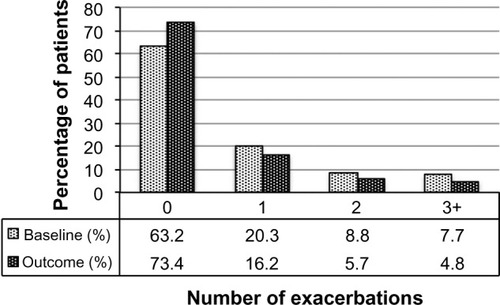
Figure 3 Acute respiratory events before (baseline) and after (outcome) addition of tiotropium. P<0.001 (marginal homogeneity test) comparing baseline and outcome years.
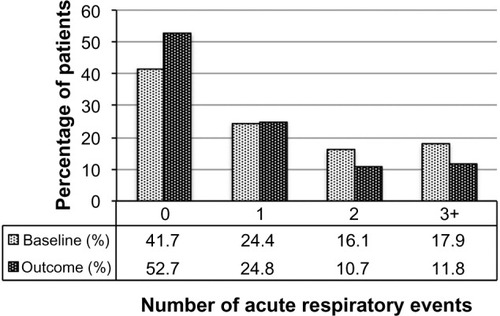
Figure 4 SABA usage before (baseline) and after (outcome) addition of tiotropium. Dosages are in salbutamol (albuterol) equivalents; P=0.006 (marginal homogeneity test) comparing baseline and outcome years.
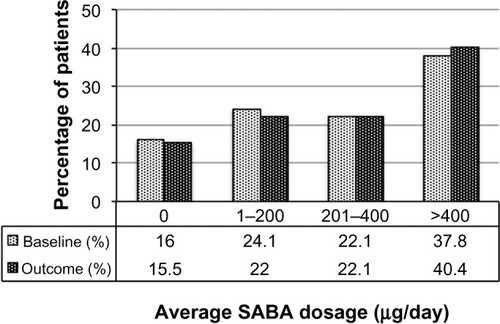
Table 4 Sensitivity analysis: baseline patient characteristics
Table 5 Sensitivity analysis: comparison of primary measures before (baseline) and after (outcome) addition of tiotropium
Table S1 Baseline variables examined
Table S2 Baseline patient characteristics: demographic and clinical variables
Table S3 Baseline smoking status by age group
Table S4 Distribution of baseline FEV1/FVC ratio in patients over 60 years of age, by smoking status
Table S5 Baseline patient characteristics: asthma treatment and control
Table S6 Comparison of effectiveness measures before (baseline) and after (outcome) addition of tiotropium
Table S7 Sensitivity analysis: comparison of primary measures before (baseline) and after (outcome) addition of tiotropium
