Figures & data
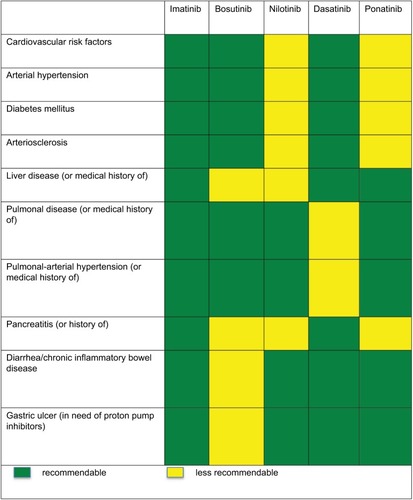
Figure 1 Study 200: Patient cohorts (n=570) in second+ line.
Abbreviations: ALL, acute lymphocytic leukemia; AP, accelerated phase; BP, blastic phase; CML, chronic myeloid leukemia; CP, chronic phase; MCyR, major cytogenetic response.
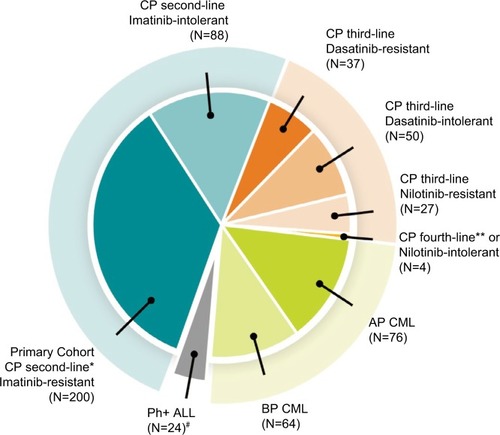
Table 1 Most frequent side effects of all approved TKI
Table 2 Efficacy parameters of bosutinib, nilotinib and dasatinib in first- and second-line treatment
Figure 2 Recommendations for TKI treatment with regard to comorbidity status.