Figures & data
Figure 1 Normal hemostasis involves both coagulation and fibrinolysis regulated by several physiologic inhibitors. (A) Phases of a cell-based model of coagulation include “initiation” that occurs after exposure of TF in the presence of VIIa, a small amount of which is normally present in the circulation, followed by “priming” in which FXa produces a small amount of thrombin to activate FVIII, FV; and platelets, resulting in “propagation”, involving assembly of activated coagulation factors on aggregating platelets to produce a thrombin burst on the surface of platelets that mediate conversion of fibrinogen to fibrin. Three endogenous anticoagulation systems modulate coagulation to prevent clot formation in excess (thrombosis) beyond the site of injury. These are predominately localized to expression on vascular endothelial surfaces. Trauma-associated coagulopathy is related to excess thrombomodulin (TM) expression resulting in high concentrations of activated protein C (aPC) and possibly to disruption of the endothelial glycocalyx with release of heparin-like proteoglycans that interact with antithrombin in a process referred to as auto-heparinization. A role for TFPI in trauma-associated coagulopathy has not been identified; however, experimentally, depletion of TFPI predisposes to disseminated intravascular coagulation, which has been suggested to be a mechanism of coagulopathy following trauma. (B) Fibrinolytic pathway and endogenous mediators that regulate fibrinolysis. Plasmin is generated from plasminogen by tPA and is controlled by several inhibitors, principally PAI-1. Upregulation of TM and thrombin–TM interactions increases thrombin-mediated activation of TAFI 1,000-fold; elevated levels of aPC may promote fibrinolysis by limiting thrombin production and consequently TAFI activation. Hyperfibrinolysis associated with trauma is thought to occur after endothelial cell perturbation following injury with trauma-induced release of large amounts of tPA in association with downregulation of PAI-1. Fibrinolysis shutdown is thought to occur through trauma-mediated dysregulation of PA-1 expression.
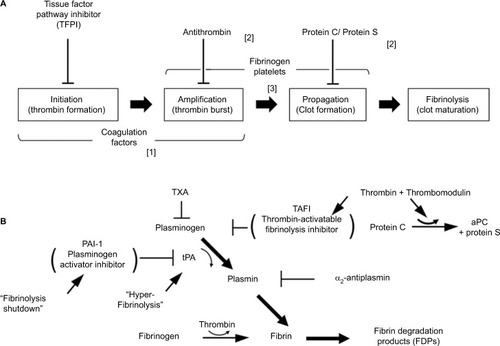
Figure 2 Thromboelastography (TEG®). (A) Schematic presentation of different viscoelastic tracings reflecting states of the coagulation system compared with normal. (B) Basic viscoelastic tracing with measured parameters and limits of normal for thromboelastography, correlated with different elements of the coagulation system (R = reaction time, K = clot formation time, angle, MA = maximum amplitude, Ly30 = percent clot lysis 30 m after MA). Viscoelastic k-time and angle correlate to some degree with fibrinogen concentration. However, the agreement between these parameters and fibrinogen levels determined by standard von Clauss assay is not sufficiently strong to be useful clinically.Citation174–Citation176 To overcome this limitation with TEG, the specific contributions of fibrinogen and platelets to clot strength can be determined with additional reagents (TEG; Functional Fibrinogen [Haemonetics Corp, Niles, IL, USA]).Citation177 Using TEG, additional measures of clot strength can be computed. Coagulation index (CI; black arrow) is derived from R, k-time, angle, and MA, with a CI > +3.0 suggesting a hypercoagulable state and CI <–3.0 suggesting coagulopathy. The shear elastic module strength, designated G, is a computer-generated quantity that reflects an integrated measure of clot strength. Conceptually, G is considered the most informative parameter of clot strength because it reflects the contributions of the enzymatic and platelet components of hemostasis.Citation178,Citation179 (C) Thrombus generation velocity curve is a mathematical first derivative of the TEG tracing, which provides additional information with respect to both thrombus formation and lysis.Citation179 For example, velocity curve measures of fibrinolysis are stronger predictors of early transfusion of blood components, bleeding, and mortality after trauma compared with conventional rTEG values. In addition, the maximal rate of lysis is more rapidly available after arrival, which may facilitate earlier diagnosis and treatment of clinically significant hyperfibrinolysis.Citation180
![Figure 2 Thromboelastography (TEG®). (A) Schematic presentation of different viscoelastic tracings reflecting states of the coagulation system compared with normal. (B) Basic viscoelastic tracing with measured parameters and limits of normal for thromboelastography, correlated with different elements of the coagulation system (R = reaction time, K = clot formation time, angle, MA = maximum amplitude, Ly30 = percent clot lysis 30 m after MA). Viscoelastic k-time and angle correlate to some degree with fibrinogen concentration. However, the agreement between these parameters and fibrinogen levels determined by standard von Clauss assay is not sufficiently strong to be useful clinically.Citation174–Citation176 To overcome this limitation with TEG, the specific contributions of fibrinogen and platelets to clot strength can be determined with additional reagents (TEG; Functional Fibrinogen [Haemonetics Corp, Niles, IL, USA]).Citation177 Using TEG, additional measures of clot strength can be computed. Coagulation index (CI; black arrow) is derived from R, k-time, angle, and MA, with a CI > +3.0 suggesting a hypercoagulable state and CI <–3.0 suggesting coagulopathy. The shear elastic module strength, designated G, is a computer-generated quantity that reflects an integrated measure of clot strength. Conceptually, G is considered the most informative parameter of clot strength because it reflects the contributions of the enzymatic and platelet components of hemostasis.Citation178,Citation179 (C) Thrombus generation velocity curve is a mathematical first derivative of the TEG tracing, which provides additional information with respect to both thrombus formation and lysis.Citation179 For example, velocity curve measures of fibrinolysis are stronger predictors of early transfusion of blood components, bleeding, and mortality after trauma compared with conventional rTEG values. In addition, the maximal rate of lysis is more rapidly available after arrival, which may facilitate earlier diagnosis and treatment of clinically significant hyperfibrinolysis.Citation180](/cms/asset/2249eafb-d80b-4c52-8fd5-5b1a7f95b8e1/djbm_a_165394_f0002_b.jpg)
Figure 3 Complement–contact activation system interactions. Coagulation factor XII-induced increases in vascular permeability and proinflammatory activity are mediated by complement activation and are regulated in part by C1 inhibitor (C1INH).Citation181 Attenuation of inflammation by C1INH has been described in several animal models of ischemia-reperfusion injury.Citation182
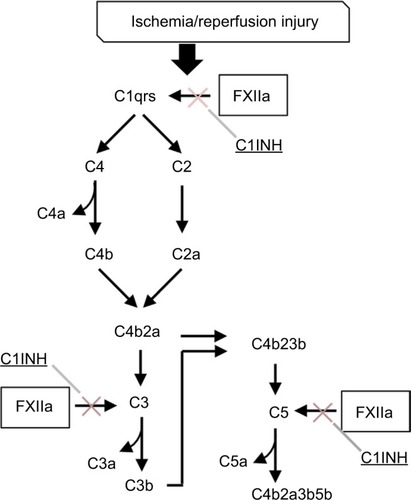
Table 1 Representative examples of recently completed or ongoing clinical trials in trauma and critical care
