Figures & data
Figure 1 Open-label study design
Note: aFor both recombinant activated factor VII-treated groups, two bolus rescue doses (90 mcg/kg) were permitted during any 24-hour period.
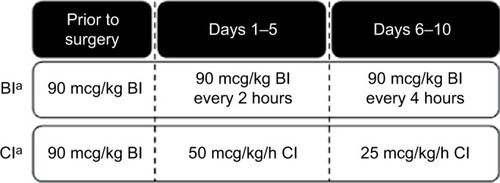
Table 1 Open-label study – efficacy of bolus dosing vs continuous infusion in major surgery in patients with congenital hemophilia with inhibitors
Table 2 Open-label study – dosing by treatment group in patients with congenital hemophilia with inhibitors
Table 3 Postmarketing surveillance study – dosing and efficacy of continuous infusion of rFVIIa in patients with congenital hemophilia with inhibitors
Table 4 Prospective studies, retrospective studies, and case reports – dosing and safety of continuous infusion of rFVIIa in patients with congenital hemophilia with inhibitors
Table 5 Prospective studies, retrospective studies, and case reports – dosing and safety of continuous infusion of rFVIIa in patients with congenital factor VII deficiency
Table 6 Summary of continuous infusion-treated events in patients with congenital hemophilia with inhibitors and congenital factor VII deficiency
