Figures & data
Figure 1 Comparison of ABR and proportion of patients with zero bleeds between BAY 94-9027 and (A) rFVIIIFc; (B) BAX 855; (C) rAHF-PFM-2004; (D) rAHF-PFM-2012. *P<0.05.
Abbreviations: ABR, annualized bleeding rate; rAHF-PFM, recombinant antihemophilic factor–plasma/albumin-free method; rFVIIIFc, recombinant factor VIII–Fc fusion protein.

Table 1 Observed baseline characteristics of patients treated with BAY 94-9027 and rFVIIIFc, before and after matching
Table 2 Observed baseline characteristics of patients treated with BAY 94-9027 and BAX 855, before and after matching
Table 3 Observed baseline characteristics of patients treated with BAY 94-9027 and rAHF-PFM
Figure 2 Comparison of weekly rFVIII utilization between BAY 94-9027 and (A) rFVIIIFc; (B) BAX 855; (C) rAHF-PFM-2004; (D) rAHF-PFM-2012. Median weekly rFVIII utilization of prophylactic BAX 855 was estimated using the median dose per infusion (44.6 IU/kg) multiplied by the median of prophylaxis infusions per week (1.96). Median weekly rFVIII utilization of rAHF-PFM-2004 was estimated using the median annualized consumption (5,733.3 IU/kg/year) multiplied by 7/365.25. Median weekly rFVIII utilization of rAHF-PFM-2012 was estimated using the median dose per infusion (30.7 IU/kg) multiplied by the protocol-specified number of prophylaxis infusions per week (ie, every other day). *P<0.05.
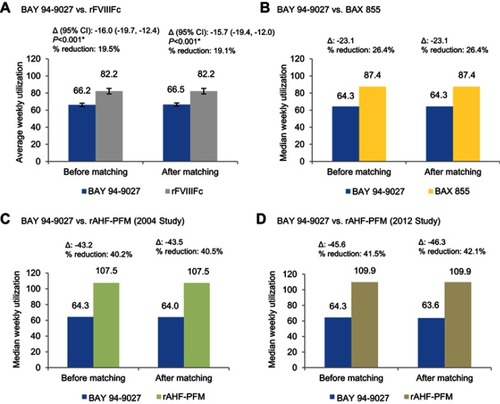
Figure 3 Relative reduction in annual IU utilization and incremental ABR and percentage of patients with zero bleeds, BAY 94-9027 vs rFVIIIFc, BAX 855, rAHF-PFM-2004, or rAHF-PFM-2012. *P<0.05.
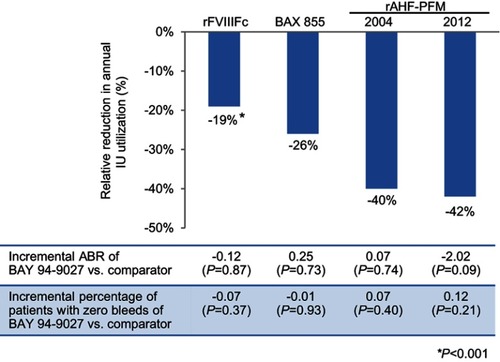
Table S1 Inclusion and exclusion criteria for the SLR
Table S2 Summary of clinical trials used in the analysis