Figures & data
Table 1 Published NBEAL2 Variants in GPS Patients
Table 2 Abnormalities in Innate and Acquired Immunity in GPS Patients and Animal Models
Figure 1 NBEAL2 variants in GPS patients. Schema of the NBEAL2 protein comprising the ARM (armadillo)-like domain, spanning amino acids (aa) 377–504, the Con (concanavalin) A-like lectin domain (aa 580–730), the PH (pleckstrin homology) domain (aa 1915–2040), the BEACH (beige and Chediak-Higashi syndrome domain (aa 2053–2345) and WD40 repeat domains (2463–2722), according to Sims et al.Citation8 Published missense, nonsense, frameshift, indel and splicing germline NBEAL2 variants described in GPS pedigrees () are indicated by the symbols. Missense variants are enriched within the BEACH domain. The position of splicing variants is given according to the predicted effect at the protein level.
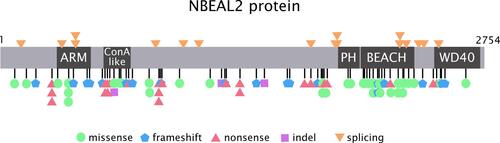
Figure 2 Pale platelets on the blood smear from a GPS patient. Large pale platelets due to absence or marked decrease in α-granules is a typical finding in GPS patients. A May-Grünwald Giemsa-stained blood smear from a GPS patient is shown on the right panel. Gray platelets are indicated by the arrows. A blood smear from a healthy subject stained simultaneously is shown on the left panel. Images were obtained at 1000x magnification.
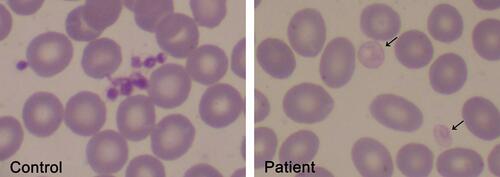
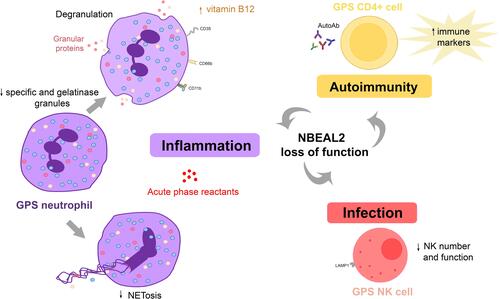
Figure 3 Abnormalities in the innate and adaptive immune response in GPS. GPS neutrophils display reduced numbers of specific (red circles) and gelatinase (yellow circles) granules. Whereas azurophilic (blue circles) granules are preserved in GPS patients, they are decreased in GPS animal models. Proteins resident in specific and gelatinase granules are expressed at the cell membrane, such as CD11b, CD66b and CD35, and elevated in plasma, indicating degranulation. Neutrophil extracellular trap formation (NETosis) is impaired. NK cell number and function are decreased in GPS animal models, although it is unknown whether it is also altered in patients. Altogether, these abnormalities may contribute to higher susceptibility to infections. Autoimmune manifestations and/or autoantibodies (AutoAb) are present in around half of GPS patients, coupled to upregulation of immune response markers in CD4+ cells. Liver-derived acute phase reactants are elevated in patient circulation, reflecting ongoing inflammation.