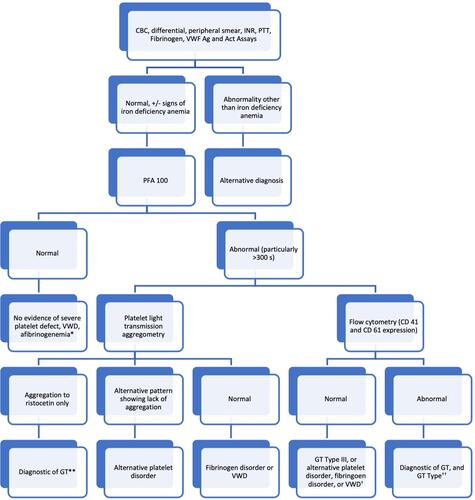
Figures & data
Figure 1 Schematic of αIIbβ3 integrin composed of αIIb and β3 subunits. The mature αIIb subunit contains extracellular heavy and light chains linked together via disulfide bridge. Both subunits contain extracellular, transmembrane, and cytoplasmic domains; the latter domains are linked via salt bridge.
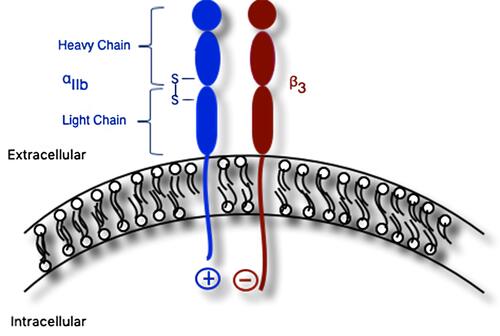
Figure 2 Schematic of αIIbβ3 integrin undergoing inside-out and outside-in signaling. (A) Bent confirmation of αIIbβ3 integrin with intact salt bridge linking cytosolic domains of the subunits (low affinity for binding fibrinogen). (B) Binding of intracellular protein talin disrupts salt bridge and triggers separation of the cytosolic region of β3 from that of αIIb, resulting in a conformational change of the αIIbβ3 integrin into the upright position. In this position, fibrinogen is able to bind extracellular domains (high affinity for binding fibrinogen; inside-out signaling). (C) Fibrinogen, in turn, binds additional αIIbβ3 integrins to facilitate platelet aggregation, resulting in activation and recruitment of additional intracellular and cytosolic proteins, such as c-Src tyrosine kinase (c-Src), integrin-linked kinase (ILK), spleen tyrosine kinase (Syk), protein kinase C (PKC), and protein tyrosine phosphatase (PTP1B) and others, to facilitate processes including cytoskeletal reorganization for platelet spreading, clot stabilization, and clot retraction (outside-in signaling).
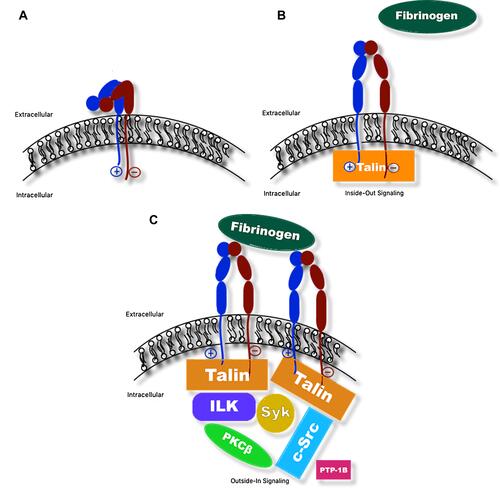
Table 1 Types of Glanzmann Thrombasthenia and Their Frequencies, with Examples of Genetic Mutations
Table 2 Clinical Presentations of Glanzmann Thrombasthenia
Figure 3 Diagnostic algorithm for GT. *Consider proceeding to platelet light transmission aggregometry if suspicion for platelet defect remains high. **Consider genetic testing to identify specific mutation of ITGA2B and ITGB3 and/or flow cytometry to differentiate GT type. †Consider clot retraction assay (if available) and platelet light transmission aggregometry or genetic testing of ITGA2B and ITGB3 to make the diagnosis of GT Type III. ††Consider genetic testing to identify specific mutation of ITGA2B or ITGB3.