Figures & data
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of the study selection process.
Note: Adapted from Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.Citation37
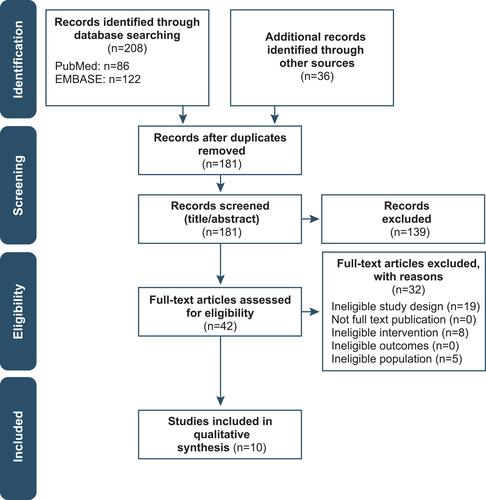
Table 1 Design and Patient Flow of Included Studies
Table 2 Baseline Patient Characteristics of Included Studies
Figure 2 Risk of bias summary: authors’ judgment about risk of bias for each item for each of the 6 included studies. The symbol “+” indicates low risk of bias, “?” indicates unclear risk of bias, and “–” indicates high risk of bias.
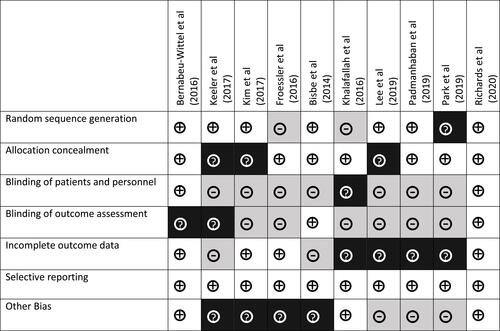
Figure 3 Risk of bias: authors’ judgment about risk of bias presented as percentages across all 6 included studies.
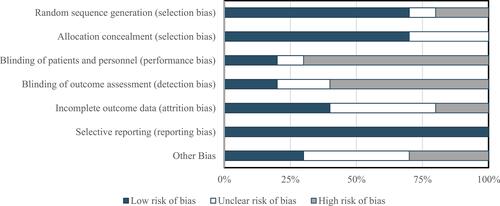
Table 3 Primary Outcome Measures: Change from Baseline in Hb, Serum Ferritin, and TSAT
Table 4 Secondary Outcome Measures: ABT, AEs, and Mortality
Table 5 Secondary Endpoint Outcomes: Hospital Length of Stay and QOL
Table 6 Quality of Evidence (GRADE) Assessment
