Figures & data
Table 1 Guidelines for Dosage During the Efficacy Component77
Figure 1 Study design.
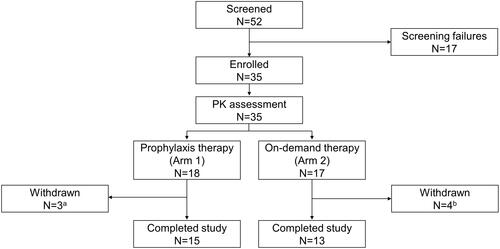
Table 2 Patient Characteristics
Table 3 PK Parameters of FVIII:C in Patients <6 (N=15) and 6–12 Years of Age (N=16)
Figure 2 Mean (SD) concentration profiles (IU/mL) of FVIII:C by age group and overall.
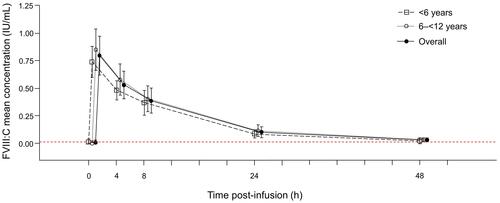
Table 4 Investigator’s Assessment of Hemostatic Efficacy per Bleeding Event in the On-Demand and Prophylaxis Arms
Table 5 Annualized Spontaneous Bleeding Rate and Prophylaxis Schedule (Number of Doses per Week per Individual Patient Who Completed the Study)
