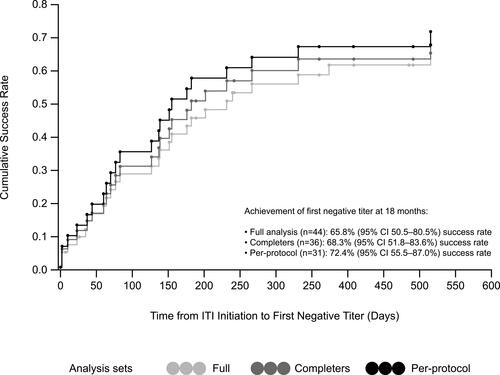
Figures & data
Figure 1 Flow of patients through the PAIR study. *Within 33 months of treatment. †As defined by the protocol.
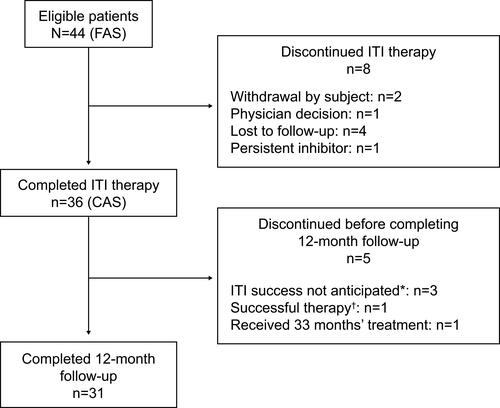
Table 1 Baseline Characteristics of Patients in the PAIR Study
Table 2 ITI Therapy Dosage Regimens Utilized in the PAIR Study
Table 3 Summary of AEs in the PAIR Study (FAS)
Table 4 Outcomes in Patients Who Completed rAHF ITI Therapy (CAS)