Figures & data
Figure 1 Study design. In this retrospective US chart review of adults diagnosed with PNH, patients needed to have been treated with a C5 inhibitor (eculizumab or ravulizumab) for at least 12 consecutive months. For patients who were switched from eculizumab to ravulizumab, patients needed to have been treated with ravulizumab for at least 6 consecutive months. The follow-up period was defined as the window of time directly preceding data entry, treatment discontinuation, or patient death. For all variables except transfusion, the follow-up period was 6 months. Transfusion follow-up was assessed over 12 months in patients treated with eculizumab or ravulizumab only and over 6 months for patients in the switch subgroup. C5, complement protein C5; PNH, paroxysmal nocturnal hemoglobinuria.

Table 1 Demographics and Disease Characteristics Prior to the Initiation of Any C5 Inhibitor
Table 2 Treatment Characteristics
Table 3 Change in Laboratory Values Between Baseline and Follow-Up (Routine Visits)
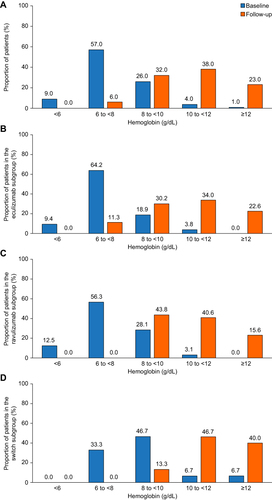
Figure 2 Improvement in hemoglobin levels with C5 inhibitor treatment. Percentage of patients by hemoglobin category at baseline and during follow-up (last 6 months of treatment with eculizumab or ravulizumab). Patients needed to have been treated with a C5 inhibitor (eculizumab or ravulizumab) for at least 12 consecutive months. For patients who were switched from eculizumab to ravulizumab, patients needed to have been treated with ravulizumab for at least 6 consecutive months. (A) All patients (N=100); data missing for three patients at baseline and one patient at follow-up. (B) Eculizumab subgroup (n=53); data missing for two patients at baseline and one patient at follow-up. (C) Ravulizumab subgroup (n=35). (D) Switch subgroup (n=15); data missing for one patient at baseline.