Figures & data
Figure 1 Enzymatic activities of wild type and mutated IDH enzymes.
Notes: The IDH family of enzymes comprises three proteins located in the cytoplasm and peroxysomes (IDH1), and mitochondria (IDH2 and IDH3). IDH1 and IDH2 catalyze the reversible NADP+-dependent oxidative decarboxylation of isocitrate to αKG. IDH3 catalyzes the NAD+-dependent conversion of isocitrate to αKG in the TCA cycle. IDH1 and IDH2 mutant enzymes gain neomorphic enzymatic activity, converting NADPH and αKG to NADP+ and D-2HG. D-2HG acts as a weak competitive inhibitor of αKG-dependent dioxygenases. αKG-dependent dioxygenases are involved in various cellular processes such as hypoxia, angiogenesis, maturation of collagens of the extracellular matrix, and regulation of epigenetics. Excess of D-2HG is associated with increased histone and DNA methylation, altering cancer cells differentiation.
Abbreviations: αKG, alpha ketoglutarate; D-2HG, D-2-hydroxyglutarate; IDH, isocitrate dehydrogenase; DNA, deoxyribonucleic acid; mut, mutated; NAD, nicotinamide adenine dinucleotide; NADP, nicotinamide adenine dinucleotide phosphate; TCA cycle, tricarboxylic acid cycle.
Abbreviations: αKG, alpha ketoglutarate; D-2HG, D-2-hydroxyglutarate; IDH, isocitrate dehydrogenase; DNA, deoxyribonucleic acid; mut, mutated; NAD, nicotinamide adenine dinucleotide; NADP, nicotinamide adenine dinucleotide phosphate; TCA cycle, tricarboxylic acid cycle.
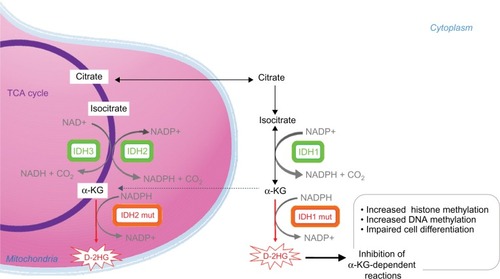
Table 1 IDH mutations estimates in solid tumors and hematologic malignancies
Table 2 Ongoing clinical trials evaluating IDH inhibitors
