Figures & data
Figure 1 Study flowchart for adults and adolescents enrolled into A-Long studies.
Abbreviation: PK, pharmacokinetic.
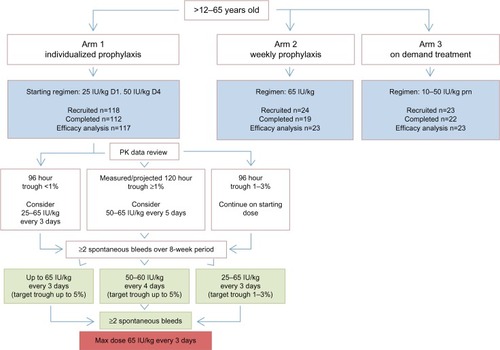
Figure 2 Study flowchart for children enrolled into A-Long studies.
Abbreviation: PK, pharmacokinetic.
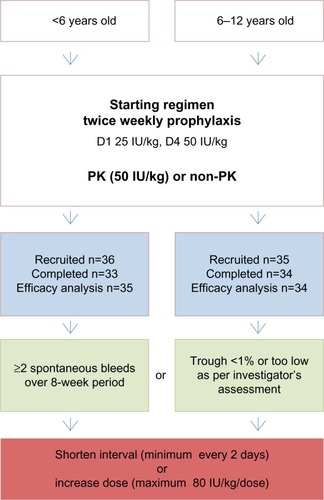
Table 1 Sampling schedule used across different arms of the study
Table 2 PK results of rFVIIIFc (efraloctocog alfa, Eloctate®) and rAHF-PFM (Advate®)
Figure 3 Comparison of rFVIIIFc and prestudy rFVIII PK parameters in pediatric study.
Abbreviations: PK, pharmacokinetic; rFVIIIFc, recombinant FVIII Fc fusion protein; CL, clearance; IR, incremental recovery; t½, half-life; pdFVIII, plasma-derived FVIII; Vss, volume of distribution at steady state.
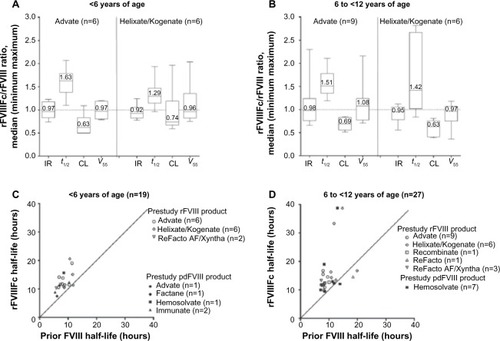
Table 3 Key outcome data relating to bleeding episodes and weekly consumption of rFVIIIFc
Table 4 Final dosing schedule at end of Phase III pivotal study illustrating the interindividual variability in prophylactic dosing regimensTable Footnotea
