Figures & data
Table 1 Superior Response to Combination of Clofarabine/Cyclophosphamide/Etoposide in Pediatric Patients with Relapsed and Refractory AML in Phase I TrialsCitation7
Table 2 FDA approved purine /pyrimidine anti-metabolites used in the combination therapy of AMLCitation6
Table 3 A Summary of Anti-AML Drugs, Targets and Mechanisms of Action
Figure 1 Summary of agents that target genome, epigenome, and signal transduction pathways in a cancerous hematopoietic cell. Inhibition of HDACs and TKRs in cancer cells displays anti-proliferative and pro-apoptotic activities in drug-resistant in vivo tumor models. The mechanisms of deoxynucleoside anti-metabolites are quite similar. They are converted to their respective nucleotide analogues which inhibit DNA synthesis, repair and methylation by inhibition of DNA polymerases and DNMTs besides inducing DNA damages by incorporation into DNA. The incorporation of these agents into DNA is difficult to repair and causes a lasting inhibition of DNA synthesis or disruption of DNA function. HDACi increases the chromatin accessibility for DNA-damaging agents and Top II inhibitors and down-regulation of DNA repair.
Abbreviations: HDACs, histone deacetylases; TKRs, tyrosine kinase receptors; FLT3, FMS-like tyrosine kinase 3; ITD, FLT3-internal tandem duplications (FLT3-ITD mutations in or near the juxtamembrane domain); TKD, FLT3-tyrosine kinase domain mutations; DNMTs, DNA methyltransferase; SAHA, vorinostat; TSA, trichostatin A; VPA, valproic acid; PB, phenylbutyrate; HMAs, hypomethylating agents.
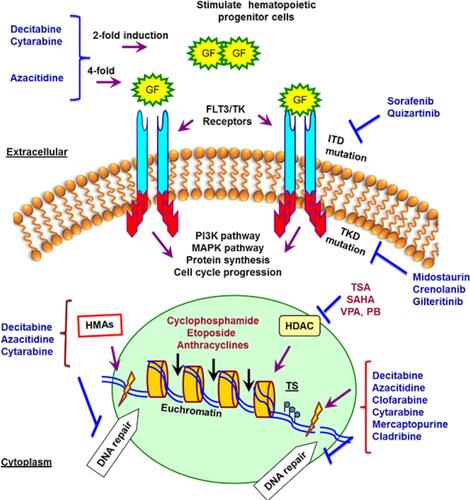
Figure 2 Targeting DNA and its repairing pathways by chemotherapy agents in AML. Therapeutics agents commonly impart their clinical efficacy via the induction of DNA damage or targeting DNA repair factors directly or indirectly. The repair of DNA damage relies on particular pathways to remedy specific types of damage to DNA. The range of insults to DNA includes small, modest changes in the structure including mismatched bases and simple methylation events to oxidized bases, intra- and interstrand DNA crosslinks, DNA double-strand breaks and protein–DNA adducts. HDACi causes down-regulation of dsDNA-break repair by affecting components of both NHEJ and HR repair pathways. Anthracyclines are widely used with deoxynucleoside antimetabolites where they exert their anti-leukemia effects through interacting with Top II and inhibiting replication and repair of DNA in leukemia cells, respectively. HDACi increases the chromatin accessibility for DNA-damaging agents and Top II inhibitors and down-regulation of DNA repair. Cisplatin and cyclophosphamide form intra- and interstrand adducts and leading to cross-linking of DNA.
Abbreviations: AML, acute myeloid leukemia; NER, nucleotide excision repair; BER, base excision repair; HDR, homology-directed repair; FA, Fanconi anemia; HR, homologous recombination; DDR, DNA damage response; NHEJ, non-homologous end joining; SSBs, single-strand breaks; DSB, double-strand breaks; PARP, poly(ADP-ribose) polymerase; NAD+, nicotinamide adenine dinucleotide; PARPi, PARP inhibitors; AMLXP, xeroderma pigmentosum; ATM, ataxia telangiectasia mutated kinase; ATR, ATM-Rad3 related kinase; Chk, checkpoint kinase; DNA pol, DNA polymerase; DNA-PK, DNA-dependent protein kinase; Top II, topoisomerase II; HDACi, Histone deacetylase inhibitors.
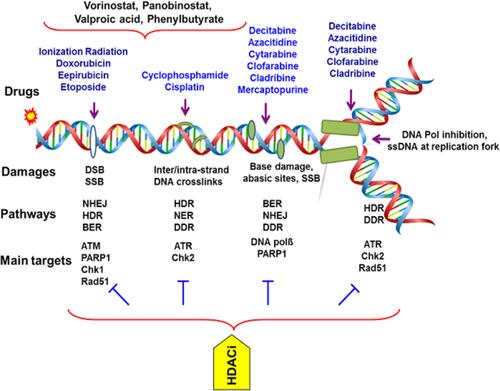
Figure 3 The sequence-specific administration of HDAC and DNMT inhibitors prior to topoisomerase II–inhibitor administration is of an utmost importance in the chemotherapy of AML. Concurrently and continuously administration of HDACi and DNMTi before chemotherapy sensitizes tumor cells to genotoxic agents, and synergistically increases expression of silenced tumor suppressors and promotes cell death and differentiation. Cisplatin, doxorubicin, etoposide are DNA-damaging agents and topoisomerase II (TOP II) inhibitors which induce DSB in the DNA of cancer cells and induce cytotoxic effects.
Abbreviations: HDACi, histone deacetylase inhibitors; DNMTi, DNA methyltransferase inhibitors; DSB, DNA strand breaks.
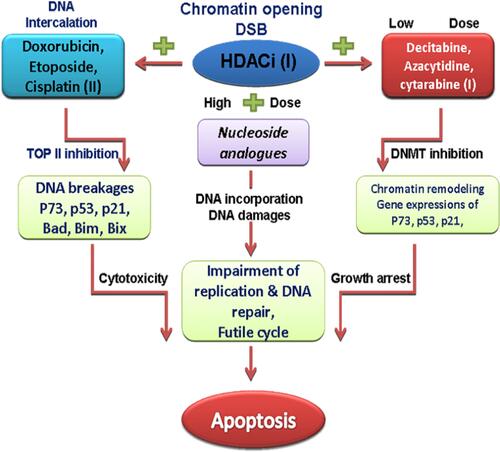
Table 4 Conventional Chemotherapy of Leukemia and Rational Chemotherapy Combinations with Histone Deacetylase Inhibitors in Phase II/III Clinical Trials.Citation14
Figure 4 Histone deacetylase inhibitors and mechanism of action which have shown benefits in early-phase clinical trials for the treatment of myeloproliferative disorders (eg, MDS and AML). Vorinostat, panobinostat and valproic acid are pan-HDAC inhibitors.
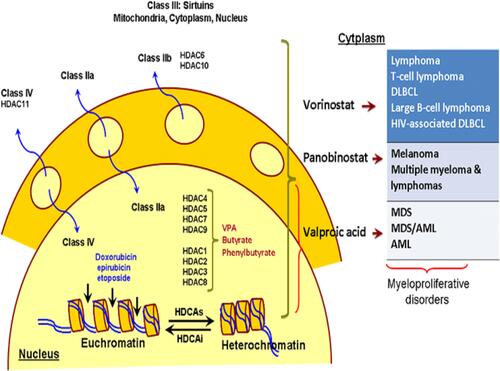
Table 5 Rational Combinations of Histone Deacetylase Inhibitors: Hydroxamic Acid-Based HDAC Inhibitors, The Zinc-Dependent HDAC Inhibitors, in Phase II/III Clinical Trials.Citation14,Citation23,Citation28,Citation29,Citation32
Table 6 Rational Combinations of Histone Deacetylase Inhibitors: Benzamide- and Short-Chain Fatty Acid-Based HDAC Inhibitors, The Zinc-Dependent HDAC Inhibitors, in Phase II/III Clinical Trials.Citation14,Citation23,Citation28,Citation29,Citation32
