Figures & data
Table 1 Plasma Biochemical Measurements in Polymyxin E (PolyE)-Treated Mice
Figure 1 Kidney tissue markers of oxidative stress in polymyxin E (PolyE)-treated mice and the effect of curcumin (CUR) treatment. Data are given as mean ± SD (n = 8). ***Indicates significantly different as compared with the control group (P < 0.01). aIndicates significantly different as compared with the PolyE group (P < 0.05).
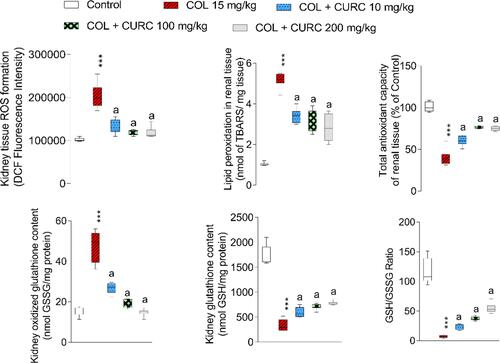
Table 2 Grade of Kidney Tissue Histopathological Alterations in Polymyxin E (PolyE)-Treated Mice
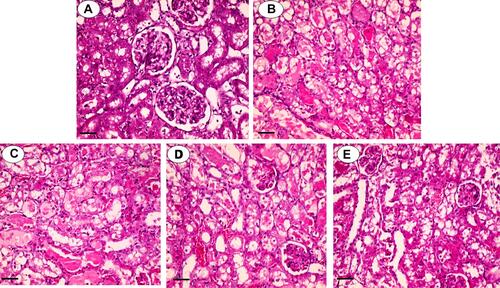
Figure 2 Kidney histopathological alterations in polymyxin E (PolyE)-treated animals. Typical kidney tissue histopathology (A). Tubular necrosis, interstitial inflammation, and atrophy were evident in PolyE-treated (15 mg/kg/day, i.v, seven consecutive days) animals (B). It was found that curcumin administration significantly decreased PolyE-induced renal injury (PolyE + curcumin; (C–E) for 10, 100, and 200 mg/kg of curcumin, respectively). The grades of kidney histopathological alterations are given in .