Figures & data
Figure 1 Cross section of an eye illustrating aqueous humor (AH) pathways (left) and site of action of antiglaucoma medications (right). AH formation occurs in the ciliary body and flows from the posterior chamber through the pupil to the anterior chamber angle. The drainage of AH is mainly facilitated by the conventional [trabecular meshwork (TM), Schlemm’s canal and episcleral veins] pathway and the non-conventional (uveoscleral-uveovortex) pathway. The current glaucoma hypotensive medications and their sites of action are shown on the right.
![Figure 1 Cross section of an eye illustrating aqueous humor (AH) pathways (left) and site of action of antiglaucoma medications (right). AH formation occurs in the ciliary body and flows from the posterior chamber through the pupil to the anterior chamber angle. The drainage of AH is mainly facilitated by the conventional [trabecular meshwork (TM), Schlemm’s canal and episcleral veins] pathway and the non-conventional (uveoscleral-uveovortex) pathway. The current glaucoma hypotensive medications and their sites of action are shown on the right.](/cms/asset/c7ff4194-6350-4f2a-8c3c-f7f3cd54fcbc/djep_a_12167330_f0001_c.jpg)
Figure 2 Rho kinase (ROCK) structure and mechanisms of activation. (A) Two isoforms of ROCKs have been identified: ROCK 1 and ROCK 2. They consist of a kinase domain, a coiled-coil region (CCR) containing the rho-binding domain (RBD), and the carboxyl terminus. The carboxyl terminus has a pleckstrin-homology (PH) domain with an internal cysteine-rich domain (CRD). The amino acid sequences of the two ROCK isoforms show the highest similarity at the kinase domain (92%). (B) In the inactive ROCK, both PH domain and RBD domain can bind independently to the kinase region forming an auto inhibitory loop. The GDP-bound RhoA is kept inactive by sequestration with guanine nucleotide dissociation inhibitors (GDI). The guanine nucleotide exchange factor (GEF) converts the inactive GDP-bound RhoA to active GTP-bound RhoA. In contrast, GTPase activating protein (GAP) converts the active RhoA to its inactive form. Binding of the GTP-bound RhoA to RBD results in an open conformation of the kinase and frees its catalytic activity. Similarly, ROCK can be activated by arachidonic acid, which binds to its PH domain. ROCK 1 can be activated by caspase-3-mediated cleavage near the carboxyl-terminus while ROCK 2 is activated by caspase-2 and granzyme B-mediated cleavage. Adapted with permission from Wirth A. Rho kinase and hypertension. Biochim Biophys Acta.2010;1802(12):1276–1284. Copyright © 2010 Elsevier B.V. All rights reserved.Citation10
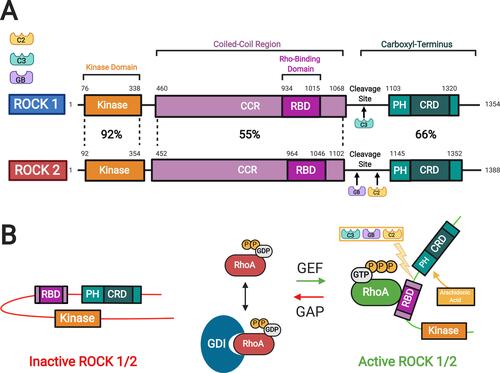
Figure 3 ROCK targets. Rho proteins can be activated by guanine nucleotide exchange factors (GEFs). GEFs are themselves activated and regulated via engagement of various receptors at the plasma membrane. The active GTP-bound Rho subsequently activates ROCK 1 and ROCK 2 that phosphorylate various substrates resulting in diverse cellular responses. The barred-line notation indicates inhibition and the arrows show the cascade of the molecular response. Adapted from Hartmann S, Ridley AJ, Lutz S. The function of rho-associated kinasesROCK1 and ROCK2 in the pathogenesis of cardiovascular disease.Front Pharmacol. 2015;6(276):1–16. Copyright © 2015 Hartmann, Ridley and Lutz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) https://creativecommons.org/licenses/by/4.0/.Citation14
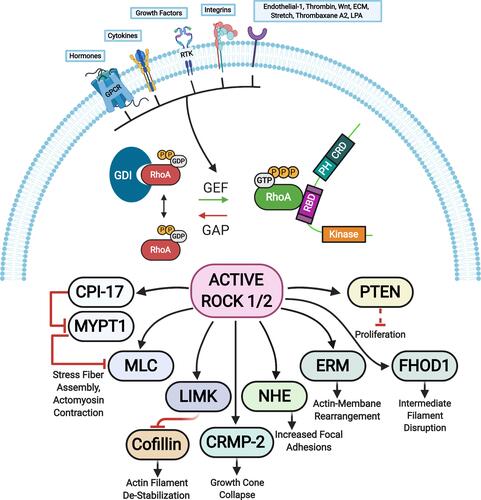
Figure 4 Therapeutic effects of Rho kinase (ROCK) inhibitors in treatment of glaucoma. They can increase aqueous humor (AH) outflow and decrease intraocular pressure (IOP) by targeting the cytoskeleton of the trabecular meshwork (TM) and Schlemm’s canal (SC) cells. ROCK inhibitors also have a neuroprotective effect by increasing blood flow to the retina and optic nerve, promoting axonal regeneration, and increasing retinal ganglion cell (RGC) survival. In addition, the inhibitory effect on Tenon fibroblasts decreases the fibrosis after filtration surgery. Finally, on the corneal endothelium they enhance cellular proliferation, promote adhesion, and suppress apoptosis. Accordingly, they provide a greater potential for the regeneration of damaged corneal endothelium and restoration of corneal transparency. The photos shown were taken at the Departments of Ophthalmology at UT Southwestern Medical Center Dallas, Texas, USA and King Hussein Medical Center Amman, Jordan. Adapted with permission from Rao PV, Pattabiraman PP, Kopczynski C. Role of the rho GTPase/rho kinase signaling pathway in pathogenesis and treatment of glaucoma:bench to bedside research. Exp Eye Res. 2017;158:23–32. © 2016 Elsevier Ltd. All rights reserved.Citation8
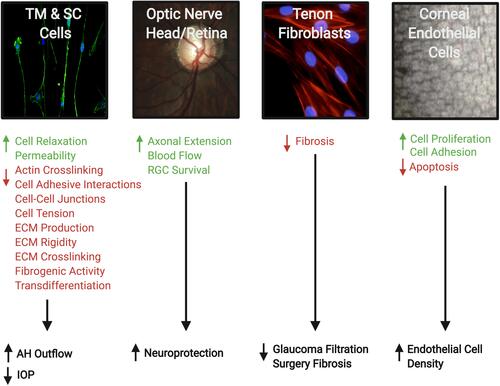
Table 1 Current Investigational Rho Kinase Inhibitors