Figures & data
Table 1 Systemic therapy approved or with positive results in phase III trials for advanced hepatocellular carcinoma
Table 2 Two major types of myeloid-derived suppressor cells and their immunosuppressive functions
Table 3 Previous clinical studies on myeloid-derived suppressor cells in hepatocellular carcinoma
Table 4 Recent preclinical studies of myeloid-derived suppressor cells in experimental hepatocellular carcinoma models
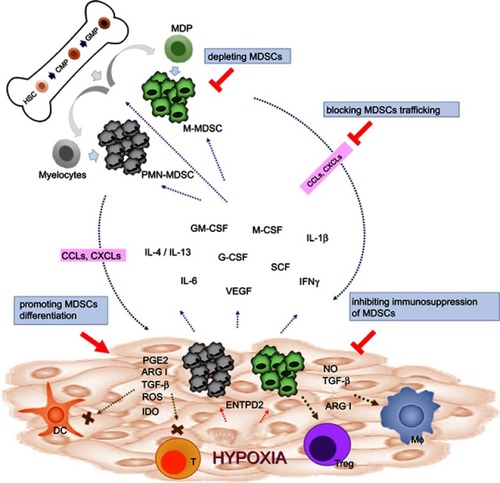
Figure 1 Strategies of targeting MDSCs in cancers. In physiological condition, HSCs differentiate into CMPs and GMPs, which subsequently become mature granulocytes or monocytes. In pathological condition such as malignancy, multiple tumor-derived factors affect the differentiation of myeloid cells, leading to the generation M-MDSCs and PMN-MDSCs. Both types of MDSCs migrate to the tumor site through the interaction of chemokine receptors and ligands (CCLs or CXCLs). In TME, MDSCs are activated and can support tumor growth by suppressing antitumor response of T cells through various mechanisms such as ARG1, iNOS, ROS, TGF-β, IL-10, and IDO. MDSCs can also promote macrophage polarization and induce Tregs and tolerogenic DCs. Reversing the protumor effects of MDSCs could be achieved by depleting MDSCs, promoting MDSC differentiation, blocking MDSC trafficking and migration into TME, and inhibiting the immunosuppressive function of MDSCs.