Figures & data
Table 1 Phase III Trials of Immune-Checkpoint Inhibitor-Based Treatment in the First-Line Setting
Table 2 Efficacy of Currently Approved First-Line Treatment Options for Advanced HCC
Figure 1 Mechanism of action of atezolizumab and bevacizumab. Atezolizumab and bevacizumab act on a positive feedback loop made of VEGF released by tumor cells and inhibitory cytokines produced by MDSCs and T-reg cells. The inhibitory cytokines in turn increase the expression of PD-L1 on tumor cells inducing immune system inhibition.
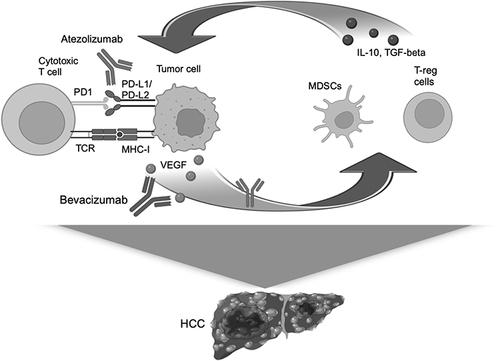
Table 3 Efficacy Outcomes of the Phase III IMbrave150 Trial (Updated Analysis)Citation11
Table 4 Safety Profile of the Combination of Atezolizumab Plus Bevacizumab Reported in the Phase III IMbrave150 Trial (Updated Analysis)Citation11
