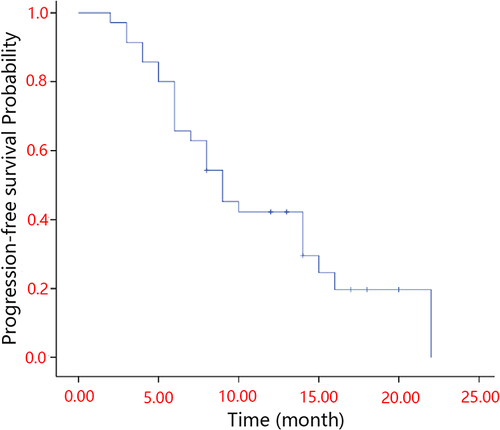
Figures & data
Figure 1 Flow diagram of patient enrollment.
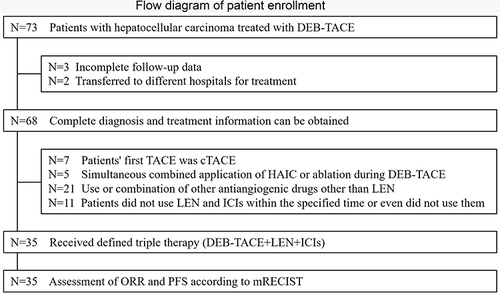
Table 1 Baseline Characteristics in 35 Patients
Table 2 Radiological Responses According to the mRECIST Criteria and Clinical Efficacy
Table 3 Tumor Responses According to the mRECIST Criteria for Different BCLC Stage
Figure 3 (a) Waterfall chart of target tumor response produced by investigator using the mRECIST criteria. (b) Tumor responses according to the mRECIST criteria for different BCLC stage.
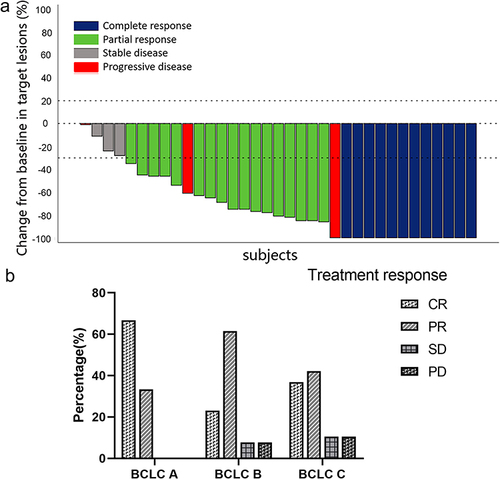
Table 4 Treatment-Related Adverse Events [n (%)]